Article Text
Abstract
Objectives Anesthesia for children with an upper respiratory tract infection (URI) has an increased risk of perioperative respiratory adverse events (PRAEs) that may be predicted according to the COLDS score. The aims of this study were to evaluate the validity of the COLDS score in children undergoing ilioinguinal ambulatory surgery with mild to moderate URI and to investigate new predictors of PRAEs.
Methods This was a prospective observational study including children aged 1–5 years with mild to moderate symptoms of URI who were proposed for ambulatory ilioinguinal surgery. The anesthesia protocol was standardized. Patients were divided into two groups according to the incidence of PRAEs. Multivariate logistic regression was performed to assess predictors for PRAEs.
Results In this observational study, 216 children were included. The incidence of PRAEs was 21%. Predictors of PRAEs were respiratory comorbidities (adjusted OR (aOR)=6.3, 95% CI 1.19 to 33.2; p=0.003), patients postponed before 15 days (aOR=4.3, 95% CI 0.83 to 22.4; p=0.029), passive smoking (aOR=5.31, 95% CI 2.07 to 13.6; p=0.001), and COLDS score of >10 (aOR=3.7, 95% CI 0.2 to 53.4; p=0.036).
Conclusions Even in ambulatory surgery, the COLDS score was effective in predicting the risks of PRAEs. Passive smoking and previous comorbidities were the main predictors of PRAEs in our population. It seems that children with severe URI should be postponed to receive surgery for more than 15 days.
- anesthetics
- patient outcome assessment
- infectious disease medicine
- pulmonary medicine
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Children with upper respiratory tract infection (URI) have an increased risk of perioperative respiratory adverse event (PRAEs).
The risk of PRAEs can be predicted by the COLDS score.
WHAT THIS STUDY ADDS
Respiratory comorbidities (adjusted OR (aOR)=6.3) and passive smoking (aOR=5.31) were predictors of PRAEs.
Patients who were canceled before 15 days had more PRAEs than the others (aOR=4.3).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The COLDS score seems to be useful in predicting PRAEs even for ambulatory surgery.
Children with severe URI symptoms should be postponed for more than 2 weeks.
Introduction
Common colds are frequent in children. It is generally due to viral infections of the upper respiratory tract. The anesthetic risk in these children is increased because of the high incidence of perioperative respiratory adverse events (PRAEs).1 This is why severely symptomatic infections with wheezing, purulent nasal discharge, fever (>38.5°C), and lethargic and ill-appearing patients should be postponed for at least 15 days. However, patients with moderate and mild symptoms, such as runny nose, sneezing, moderate cough, sore throat, and low-grade fever (<38.5°C), can be managed with caution by experienced pediatric anesthesiologists.2 The use of recent guidelines for the management of these patients can reduce anesthesia-related morbidity but cannot eliminate it. Nevertheless, predicting the risk of PRAEs in children using the COLDS score3 may facilitate the decision of whether to proceed with surgery or postpone the patient. However, the validity of this score in a homogenous population with the same age range, the same airway management, mild to moderate upper respiratory tract infection (URI), and the same surgical procedures can be compromised.
The aim of this study was to evaluate the validity of the COLDS score among children aged 1–5 years undergoing ambulatory anesthesia for ilioinguinal surgeries and suffering from a moderate URI and to investigate other predictors of PRAEs in this population.
Methods
The study was conducted in the ambulatory pediatric surgery department of the Hedi Chaker University Hospital in Sfax, Tunisia, from November 1, 2021, to April 30, 2022.
In this study, we included all children aged 1–5 years undergoing pediatric ambulatory ilioinguinal surgery who had a URI with mild to moderate symptoms such as runny nose (clear rhinorrhea), sneezing, pharyngitis (sore throat), fever of <38.5°C, rhonchi, moderate cough defined by occasional hems, and isolated and/or paroxysmal cough, without additional symptoms.4 Patients with severe symptomatic infections with wheezing, purulent nasal discharge, fever (>38.5°C), and lethargic or ill-appearing patients were canceled and postponed for 15 days from the onset of the symptoms. These canceled patients can be included in the study (15 days later) if they still have persistent mild to moderate signs of URI. We excluded patients who did not adhere to the protocol of the study and patients whose parents did not consent.
The variables included age, weight, sex, previous comorbidities, respiratory chronic diseases, and obesity (body mass index of ≥95th percentile for children of the same age and sex). Passive smoking (exposure to environmental tobacco smoke), a history of respiratory infection in the previous 15 days, and whether the patient was canceled before 15 days were noted. Furthermore, we looked into the clinical signs of a moderate URI, such as runny nose, sneezing, sore throat, 38.5°C fever, rhonchi, and moderate cough.
The COLDS score (online supplemental appendix 1) was calculated at the time of patient inclusion in the study.3 The main outcomes were the incidence of PRAEs and their impact on the ambulatory procedure. We considered ambulatory procedure failure when the patient was admitted to the hospital. The PRAEs included both perioperative and postoperative adverse events and were defined by the occurrence of one or more of the following items listed:
Supplemental material
Oxygen desaturation of <92% (SpO2 <92%) at any moment during the surgery or later in the postanesthesia care unit (PACU).
The incidence of bronchospasm included elevated peak inspiratory pressure during anesthesia (>30 cm H2O), wheezing, and oxygen desaturation.
Incidence of laryngospasm.
Copious secretions requiring endotracheal suctioning during anesthesia.
Severe, strenuous cough, accompanied by chest discomfort or abnormal breath sounds after anesthesia.
Need for prolonged oxygen support (>1 hour postoperatively) to maintain SpO2 of >95%.
Need for nebulizer in the PACU (salbutamol).
The anesthesia protocol was standardized for all patients. A preanesthetic assessment was performed on the day of surgery and before ambulatory admission. Patients with severe URI symptoms were delayed for 15 days from the onset of symptoms. However, in the case of mild to moderate signs, surgery was accepted, and nurses used saline nasal drops to wash away built-up mucus, followed by inhalation of salbutamol (0.5% solution, 0.02 mL/kg, maximum 0.5 mL) diluted to a total volume of 2 mL and administered by face mask and nebulizer. After the routine anesthesia checklist, all patients had inhalation induction with sevoflurane (8%) delivered by a calibrated vaporizer through an open circuit. Children were breathing spontaneously during induction with 100% oxygen and 6 L/min gas flow under standard monitoring. After placement of an intravenous line, we administered 30 µg/kg alfentanil without muscle relaxant. When the depth of anesthesia was deemed appropriate (apnea), an I-Gel laryngeal mask airway was inserted, and the lungs were ventilated with a volume-controlled ventilator at 1.5 MAC (minimal alveolar concentration) sevoflurane (O2/air: 50%). The tidal volume was 6–8 mL/kg, and the respiratory rate was 20–30 cycles/min without PEEP to keep pEtCO2 (partial expiratory end tidal CO2) in the 30–35 mm Hg range. Pmax was fixed at 30 cm H2O. During the surgery, we used 3% sevoflurane for anesthesia maintenance. Hemodynamic and respiratory parameters, particularly volume, pressure, SpO2, and pEtCO2, were assessed. At the end of surgery, all patients received 15 mg/kg paracetamol for pain relief. Then, sevoflurane was stopped, and the I-Gel supraglottic airway device was removed when the patient was fully awake and effective spontaneous breathing had been acquired. Then, the patient was referred to the PACU for 2 hours. The modified Aldrete score,5 assessing patient activity, respiration, blood pressure, consciousness, and color, was used for patient discharge from the PACU. An Aldrete score of >9 is needed. In the ambulatory pediatric surgery unit, a Chung score of ≥9 with the absence of any breathing difficulty or abnormal breath sounds was required for hospital discharge.6 Parents were aware of the risk of hospitalization in the case of PRAEs. PRAEs were managed according to the classic guidelines.7 8
Patients included in the study were divided into two groups based on the incidence of PRAEs:
Group C (complicated): patients who presented with PRAEs.
Group NC (non-complicated): patients who had no PRAEs.
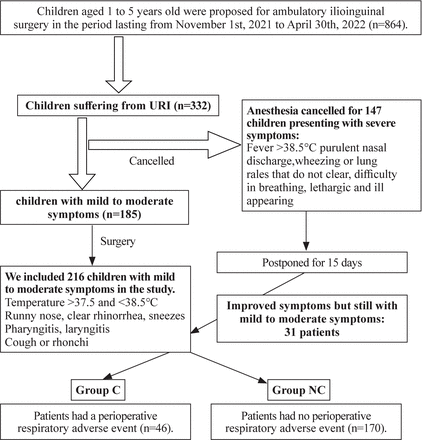
Figure 1 is a flowchart summarizing the selection of patients and the creation of the two groups and shows how the study size was arrived at.
Patients’ selection and study groups. URI, upper respiratory tract infection.
All statistical analyses were performed using the SPSS V.23.0 statistical package. Continuous variables are presented as mean±SD. We distinguished two groups according to the incidence of PRAEs. The comparison between groups was achieved by Student’s t-test and χ2 test for continuous variables and categorical variables, respectively. Univariable logistic regression analyses were used to determine crude ORs with 95% approximate CIs as estimators of PRAEs. To assess the predictors of PRAEs among children with mild to moderate symptoms of URI undergoing ambulatory pediatric surgical procedures, we performed a multivariable logistic regression model. The significance threshold was set at a p value of <0.05.
Results
Of the 864 children aged 1–5 years old scheduled for ambulatory surgical procedures in the period of the study, 332 were suffering from a cold. According to the preanesthetic assessment, 185 were considered to have a mild to moderate form of URI, and the 147 others had severe signs and were postponed for 2 weeks. Thirty-one patients from the 147 postponed patients were included in the study after 15 days, as they remained mildly symptomatic. We included 216 children. Then, patients were divided into two groups: 46 patients in group C (with PRAEs) and 170 patients in group NC (without PRAEs). Therefore, the incidence of PRAEs in this study was 21.3%.
The demographic parameters (age, weight and gender) were comparable in both groups. Patients with comorbidities, particularly respiratory comorbidities and obesity, were more frequent in group C, with p values of <0.001. In this study, we noted 17 children with previous respiratory comorbidities: 2 cases of obstructive sleep apnea, 1 case of severe asthma, and 14 cases of intermittent and moderate asthma.
The patients who were canceled before 15 days because of a severe URI were more frequent in group C (p<0.001). Passive smoking was observed in 71% of group C vs 14.1% in group NC (p<0.001) (table 1). Clinical features during the preanesthetic assessment were comparable in both groups (table 2). However, the COLDS score ranged from 8 to 15 in our population, and it was superior to 10 in 54% of the patients in group C vs 12.3% in group NC (p<0.001).
Demographic parameters and comorbidities
Clinical features during the preanesthetic assessment
PRAEs occurred during anesthesia in 36 patients (78.3%) and in the PACU in 10 patients (21.7%). PRAEs during anesthesia were bronchospasm in 32 patients: 10 in the perioperative period and 22 during awakening. A severe decrease in SpO2 (<92%) was seen in all of them. Only one case of laryngospasm was noted at anesthetic induction and required tracheal intubation. This patient developed pulmonary edema later and was referred to the pediatric intensive care unit with favorable outcomes. Copious secretions requiring endotracheal suctioning during anesthesia (with no bronchospasm) were noted in three other patients. In the PACU, 10 patients had severe coughs and abnormal breath sounds (1 wheezing and 9 rhonchi). No oxygen desaturation (<92%) was noted in the PACU. However, 40 patients needed oxygen support for more than 1 hour, and 39 needed salbutamol nebulization. Ambulatory procedures failed only in the 33 patients who had bronchospasm or laryngospasm, and all of them were admitted.
In univariable logistic regression, patients with comorbidities, respiratory comorbidities, obesity or sleep apnea, passive smoking, an URI within the preceding 2 weeks, a COLDS score of >10, and patients postponed 15 days ago were predictors of PRAEs (table 3).
Predictors of perioperative respiratory adverse events
In multivariable logistic regression, predictors of PRAEs were respiratory comorbidities (adjusted OR (aOR)=6.3, 95% CI 1.19 to 33.2, p=0.003; aOR=4.3, 95% CI 0.83 to 22.4, p=0.029), passive smoking (aOR=5.31, 95% CI 2.07 to 13.6; p=0.001), and COLDS score of >10 (aOR=3.7, 95% CI 0.2 to 53.4; p=0.036) (table 3).
Discussion
Our study showed that the COLDS score was effective in predicting the risk of PRAEs even in a homogenous population undergoing minor ambulatory surgical procedures. The other main predictors were respiratory comorbidities, passive smoking, and patients who were postponed before 15 days. Our study emphasizes the role of the COLDS score in predicting PRAEs in pediatric ambulatory surgery. Although we calculated a COLDS score for each patient, the scoring system was not used as a decision tool in determining whether or not to cancel the patient. This decision remains controversial because there are still few data about the cut-off for the COLDS score needed to cancel them.3 9 Even if it seems that this score can improve knowledge of the probability of a PRAE, which may increase awareness and initiate additional measures such as preoperative nebulizers of bronchodilators and parents’ education, the COLDS score cannot be used in the cancelation decision.
We suggest that the decision of whether to cancel surgery in children with URI should take into consideration not only the COLDS score (≥10) but also other predictors of PRAEs reported in our study.
In our population, the incidence of PRAEs was higher than that in the literature, and an urgent quality process is required to improve the outcomes.10 This may be due to including children who were canceled before 15 days because of severe respiratory infections. It seems that this delay (15 days after the onset of symptoms, according to the protocol of our department) is insufficient because children who experienced a severe respiratory upper tract infection 15 days ago may retain bronchial hyper-responsiveness (copious secretions), especially in children with respiratory comorbidities or exposed to environmental tobacco smoke. These patients need a longer period to recover fully.11 The high incidence of PRAEs can also be explained by the high rate of passive smoking in our population.12 Exposure to environmental tobacco smoke has been reported previously as a predictor of PRAEs.13 14 In fact, passive smoking inhibits lung growth during childhood and can promote asthma and airway hyper-reactivity. It reduces the immunological response of the lungs and exposes children to an increased risk of respiratory tract infections and delayed recovery.15 In our pediatric population, obesity was frequent and might be associated with some specific respiratory comorbidities, such as obstructive sleep apnea and restrictive lung disease, which can increase the risk of PRAEs.16 17 Respiratory comorbidities were the main risk factor for PRAEs according to our study, and these results were comparable with those in the literature.18 19 In our study, all patients included were homogenous. We included patients with the same age range and the same anesthetic technique for the same type of surgery. This is why these factors, considered predictors of PRAEs in a previous study,20 were comparable between the two groups in our study. The identification of the risk factors for PRAEs is very interesting in pediatric anesthesia. It can be useful for parents’ informed consent and for the decision of whether to cancel, postpone, or proceed with surgery. The COLDS score is a heuristic preanesthetic risk score that is widely used to predict the incidence of PRAEs in children with URI.3 21 This score is based on five items: the severity of current symptoms, the onset of the signs, respiratory comorbidities, airway management device, and the type of surgery.21 This score was validated,3 and we adopted it in our department. However, children undergoing ambulatory ilioinguinal surgery (1 point in the COLDS score), having mild to moderate symptoms (2 points), with an onset of signs generally between 2 and 4 weeks (2 points), needing general anesthesia and being ventilated using an I-Gel (2 points) will have a comparable COLDS score, although the risk is not comparable. In our population, the only difference in COLDS score will come from respiratory comorbidities. This may explain why the COLDS score was a predictor of PRAEs in our population. We think that predictors of PRAEs in pediatric ambulatory anesthesia need specific risk tools and specific risk scores. All risk assessment tools have limitations and should be used within an overall clinical decision-making process.22 A previous study17 validated five risk factors for PRAEs in pediatric ambulatory anesthesia: age ≤3 years, ASA physical status of >1, morbid obesity, preexisting pulmonary disorder, and surgery (vs radiology). These factors were not found in our research. We should also mention that PRAEs can result in the failure of the ambulatory procedure, and the patient may require hospital admission. Therefore, physicians should balance the risk:benefit ratio before proceeding to surgery, even if delaying elective surgery can have a negative impact on hospital finances and resources, as well as training residents.23
The main limitations of this observational study were that we focused only on the assessment of the risk factors for PRAEs in children undergoing ambulatory surgery, and we did not investigate the repeatability and reproducibility of COLDS scoring, in addition to the lack of blinding. The second limitation was that we did not study the role of our anesthetic protocol,24 which did not include regional analgesic techniques,25 in increasing the risk of PRAEs, as we used the same protocol for all patients.
In conclusion, children with URIs have an increased risk of PRAEs. Investigating predictors is essential and may help pediatric anesthetists assess the risk before any anesthesia in a child with URI, even for ambulatory minor surgery. The COLDS score, initially validated for different types of surgeries requiring different anesthetic techniques, seems to be effective in predicting the risk of PRAEs in children undergoing ambulatory surgery. Nevertheless, this study showed that particular consideration should be given to respiratory comorbidities and passive smoking. We also showed that the delay of 15 days seems to be insufficient for patients having severe URI symptoms, as the patients who were delayed 15 days had more complications than the others. Pediatric patients with mild to moderate URIs should be managed by an experienced team because the incidence of PRAEs remains high.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the local ethical committee for research in human subjects of the university of Sfax (IRB153/2021). The participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors acknowledge the Hedi Chaker University Hospital, which supported our patients and protocols.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors AJ contributed to conceptualization, methodology, resources, validation, formal analysis, data curation, software, investigation, and writing (review). MK contributed to writing (original draft), project administration and editing. SA contributed to the formal analysis and editing. WF contributed to software, investigation and editing. KK contributed to writing (review), supervision and editing and is the guarantor of the study. All authors in the article contributed and approved the submission of the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.