Article Text
Abstract
Background The estimated prevalence of esophageal atresia (EA) is 1 in 2500–4500 live births (LBs). Researchers have already identified risk factors, but the mechanisms are still unknown. The aim of this study is to identify EA prevalence trends and its risk factors in the São Paulo State (SPS) population database.
Methods We conducted a population-based study using all EA cases identified by the Live Births Information System across 14 years (2005–2018) to estimate EA prevalence trends in recent years, stratified by maternal age and SPS geographical clusters. We calculated the prevalence trends, regression coefficient (β), annual percent change (APC), and 95% confidence interval (CI).
Results We found 820 EA cases among 8,536,101 LBs with a prevalence of 1.0/10,000 LBs in SPS, Brazil. There was no significant difference in distribution by sex. Among all the cases, the majority (65%) were Caucasian; 51.8% were born at term; 43% had weight of ≥2500 g; 95.4% were singleton; and 73.4% of births were by cesarean section. From 2005 to 2018, there was an increasing trend of EA prevalence (APC=6.5%) with the highest APC of 12.2%. The highest EA prevalence rate (1.7/10,000 LB) was found in the group with maternal age of ≥35 years. No significant seasonal variation was found based on the conception month (p=0.061).
Conclusions EA had an increasing prevalence trend in SPS, Brazil, in recent years, with the highest prevalence rate in the group with maternal age of ≥35 years. No seasonality was observed. This population-based study is the first to summarize the current epidemiology of EA in SPS LB.
- Epidemiology
- Neonatology
- Statistics
Data availability statement
Data are available in a public, open access repository. The microdata used for this study is administered by the Live Births Information System (Sistema de Informação Sobre Nascidos Vivos), using data from the Unified Health System Department of Informatics (Departamento de Informática do Sistema Único de Saúde (DATASUS)), maintained by the Ministry of Health of Brazil. DATASUS provides open public access to these data for any purpose (www.datasus.saude.gov.br-http://datasus.saude.gov.br/informacoes–de–saude/tabnet/estatisticas–vitais).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is already known on this topic
The estimated esophageal atresia (EA) prevalence is 1 in 2500–4500 live births (LBs), with a higher prevalence in lower-income to middle-income countries compared with high-income ones.
What this study adds
EA prevalence in São Paulo State increased from 0.5/10,000 LBs to 1.5/10,000 LBs from 2005 to 2018 with an average rate of 6.5% per year,
It does not show monthly seasonal variation.
How this study might affect research, practice or policy
Prenatal follow-up should be distinguished between mothers <20 years and ≥35 years of age, and ultrasound examination should focus on identifying congenital malformations such as EA.
High-complexity hospitals with trained medical personnel should be established in areas far away from large urban centers to diagnose and treat congenital malformations such as EA.
Introduction
Esophageal atresia (EA) is the principal congenital defect affecting the esophagus and requires reconstructive surgery.1 2 It is a congenital anomaly that occurs between the fourth and fifth weeks of embryological development during anterior intestine formation and separation.3–5 Generally, EA is associated with other congenital malformations or abnormalities, including sequence of vertebral anomalies, anal atresia, cardiovascular anomalies, tracheosophageal fistula, renal and/or radial anomalies, and limb defects, trisomy 18 or 21, and CHARGE syndrome (coloboma, heart defects, atresia choanae, growth retardation, genital abnormalities, and ear abnormalities).6–8
The estimated prevalence of this defect is 1 in 2500–4500 live births (LBs), with a higher prevalence in lower-income to middle-income countries than that in high-income countries.9–11 The mechanisms or risk factors of EA are still unknown; some researchers have already identified risk factors, such as white race, fetus male (M) sex, multiple gestations, low birth weight (≤2500 g), prematurity (≤36 weeks), maternal age of ≤20 years or ≥35 years, and being born in urban areas.2 12
The present study aims to characterize the descriptive epidemiology and to identify EA prevalence trends and its risk factors using the São Paulo State (SPS) population database.
Methods
Study design and participants
This is a population-based study with a time trend,13 following Strengthening the Reporting of Observational Studies in Epidemiology14 guidelines. The present study was conducted in SPS, which is the most populous in Brazil, with a population (41,262,199 in 2010)15 and birth rate (610,000/year)16 comparable to countries in Europe17 18 and Latin America19 20 and where the completeness of public data is more reliable.21 22 We included all LBs of mothers residing in SPS, Brazil. Official microdata of all EA cases identified by the Live Births Information System (Sistema de Informação sobre Nascidos Vivos) in SPS, Brazil, from January 1, 2005, to December 31, 2018 were used. We extracted data on the maternal city of residence from the Unified Health System Department of Informatics15 (Departamento de Informática do Sistema Único de Saúde (DATASUS)), a department maintained by the Ministry of Health of Brazil. The 10th Edition of International Classification of Diseases (codes Q39.0, Q39.1, and Q39.2) was used23 to identify all EA cases, with or without tracheoesophageal fistula (TEF), and TEF alone, at the LB declaration, among all LBs in the period of study.
Variables
Sociodemographic and clinical explanatory variables of LBs such as gender, race/color, gestational age, birth weight, maternal age, maternal schooling, maternal occupation, type of gestation, type of delivery, and the number of prenatal consultations were all collected.We calculated EA prevalence trends according to maternal age range (≤ 14, 15–19, 20–24, 25–29, 30–34, and ≥35 years) and for territorial clusters (São Paulo Metropolitan Region (SPMR), São Paulo City (SPC), Baixada Santista Metropolitan Region (BSMR), Taubaté Administrative Area (TAR), Central South Cluster (CSC), Campinas Region Cluster (CRC), Central North Cluster (CNC), and Northwest Cluster (NWE)) in SPS, following Calderon et al’s proposal.24
Data sources
We extracted the microdata from the file transfer service provided by DATASUS. The TABNET and TABWIN programs were used to extract the data. These tabs were developed to perform fast tabulations on DBF files, and then we expanded the files to the XLS version and classified the variables selected for this study in Excel. To minimize possible discrepancies, two researchers collected data independently.
DATASUS developed the Live Birth Information System to gather epidemiological information on births reported throughout the country to subsidize interventions related to women’s and children’s health for all levels of the Unified Health System (Sistema Único de Saúde). This database has been validated and previously used for public health and perinatal epidemiological research and surveillance.24–26
Statistical analysis
We used only the population of LBs to obtain the prevalence rates to remove age bias. Therefore, it is unnecessary to standardize the rates since all the studied populations (LB) had the same age. With this, the result obtained by the analysis can be used for comparison with other studies. EA prevalence rates were calculated for 10,000 LBs by territorial clusters and maternal age group in a global period (2005–2018) and two time intervals of 7 consecutive years (2005–2011 and 2012–2018).
We used the Prais-Winsten regression model for trend analysis, following Antunes and Cardoso’s13 methodological indications. The dependent variable was the logarithm of the rates, and the independent variable was the years of the historical series. The annual percent change (APC) of the rates was also calculated, as suggested by Antunes et al.27
The data modeling process includes transforming the standardized rates into a base 10 logarithmic function using the Durbin-Watson test to measure the existence of first-order autocorrelation of the time series composed of the annual coefficients and to verify if the correlation was compatible with the random regression residuals hypothesis. According to geographical clusters and maternal age groups, annual rates of increase or decrease APC with the respective 95% confidence interval (CI) were then calculated. This procedure makes it possible to classify the EA trend as increasing, decreasing, or stationary, considering to be stationary the trend whose coefficient was not significantly different from 0 (p>0.05).13 To facilitate graphical visualization, we performed the third-order centered moving averages technique for trends and seasonality.27 28
To model seasonality, we used monthly measures for births (84 months). The calendar months were numbered sequentially for monthly measurements; for the seasonality hypothesis test, Antunes et al’s27 methodological indications were used. For seasonality analysis, we used the month of the conception date; in this case, the available data were from April 2011 to April 2018. We considered the seasonal variation significant if one or more of the coefficients of the seasonal term (B3 and B5 for Seno and B2 and B4 for Cosseno) were significantly different from 0 (p<0.05).13 We used Stata V.15.1 for all statistical analyses.
Results
Sociodemographic distributions of LBs with EA in SPS, Brazil, from 2005 to 2018 are summarized in table 1, including data on total births, type of birth, maternal and infant characteristics, with the respective p values for each variable. We identified a higher proportion of EA (52.8%) in men than in women and a greater proportion of white births (65%). The highest proportional prevalence was in preterm infants from 28 to 31 weeks (7.89/10,000 LBs, 95% CI 5.93 to 9.86).The highest proportion of EA LB had weight of ≥2500 g (43%). The highest proportional prevalence group was <1500 g (11.24/10,000 LBs, 95% CI 9.36 to 13.12). EA prevalence was found to be higher in mothers aged younger than 14 years (30.22/10,000 LBs, 95% CI 25.16 to 35.28) and more than 35 years (1.66/10,000 LBs, 95% CI 1.43 to 1.89) compared with the other maternal age groups, and in these age groups, we found the lowest and the highest proportion of cases (0.7% and 24.5%, respectively). The vast majority of cases occurred in single pregnancy (95.4%), but there was a higher proportional prevalence in twin pregnancy (1.87/10,000 LBs, 95% CI 1.26 to 2.48). Cesarean delivery was the most frequent (73.4%) and prevalent (1.2/10,000 LBs, 95% CI 1.11 to 1.3) compared with vaginal delivery in EA LBs.
Maternal and infant sociodemographic characteristics in São Paulo State, Brazil, from 2005 to 2018
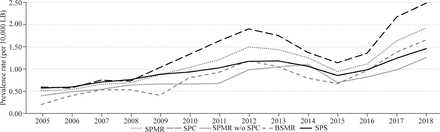
The annual proportions and prevalence trends of EA cases in LBs in all SPS clusters from 2005 to 2018 are summarized in online supplemental table 1 and figure 1. During this period, SPS presented the highest number of cases (91) and the highest prevalence rate (1.5/10,000 LBs) in 2018. From 2005 to 2018, SPC presented the highest annual prevalence rate (2018: 3.0/10,000 LBs) and total prevalence (1.3/10,000 LBs), while TAR presented the lowest total prevalence (0.6/10,000 LBs). There was an increasing EA prevalence trend in SPMR, SPC, SPMR without SPC, BSMR, and SPS from 2005 to 2018. Their increasing APC rates were 11.0%, 12.2%, 6.7%, 11.6%, and 6.5%, respectively, while the other cluster’s tendencies were stationary. In the first period (2005–2011), an increasing EA prevalence trend was observed in SPMR, SPC, SPMR without SPC, and SPS, presenting the following APCs: 18.5%, 23.7%, 9%, and 12%, respectively. In the second period (2012–2018), the EA prevalence trend was stationary in all clusters. We noticed that the TAR, CSC, CRC, CNC, and NWE clusters showed a stationary prevalence trend from 2005 to 2018 and during the two-interval periods (table 2).
Supplemental material
Esophageal atresia prevalence trend (per 10 000 LBs) by clusters in SPS, Brazil, from 2005 to 2018. BSMR, Baixada Santista Metropolitan Region; LB, live birth; SPC, São Paulo City; SPMR w/o, São Paulo Metropolitan Region without SPC; SPS, São Paulo State.
Prais-Winsten regression model for trends in EA prevalence by clusters in SPS, Brazil, from 2005 to 2018
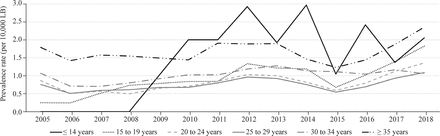
The annual EA prevalence trends in different maternal age group were also analyzed. The group with maternal age of ≤14 years had the highest EA prevalence in 2013 (5.8/10,000 LBs), while the group with maternal age of 15–19 years had the lowest EA prevalence in 2006 (0.1/10,000 LBs) (figure 2 and online supplemental table 2). From 2005 to 2018, the maternal age group of 15–19 years had the highest APC (APC=15.5%, 95% CI 3.7% to 28.6%). When separated by period, only the maternal age group of 15–19 years presented an increasing prevalence trend (APC=40.0%, 95% CI 6.6% to 83.7%) from 2005 to 2011, and all other maternal age groups presented a stationary prevalence trend. In the second period (2012–2018), all maternal age groups presented a stationary prevalence trend (table 3).
Supplemental material
Esophageal atresia prevalence trend (per 10,000 LBs) by maternal age group in São Paulo State, Brazil, from 2005 to 2018. LB, live birth.
Prais-Winsten regression model for trends in esophageal atresia prevalence, by maternal age group in São Paulo State, Brazil, from 2005 to 2018
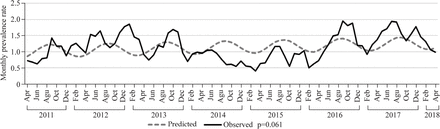
From 2011 to 2018, no significant seasonal variation in EA monthly prevalence rate was found based on the month of conception (95% CI −0.047 to 1.972, p=0.061) (figure 3).
Esophageal atresia monthly prevalence rate (per 10,000 live births) in São Paulo State, Brazil, from 2011 to 2018.
Discussion
To the best of our knowledge, this is the first population-based study to describe the recent epidemiology of LBs with EA, its seasonality, and prevalence in SPS, Brazil, in a recent period of 14 years (2005–2018). Furthermore, this study adds essential information on EA to the limited data in Brazil since this population has not been previously evaluated in detail concerning this pathology.
The EA prevalence found in SPS of 0.96/10,000 LBs was lower than that found in many previous studies with a range of 1.8–11.98/10,000 LBs.2 29–35 We found only one study with the EA prevalence similar to ours, carried out by Rankin et al,36 in five British regions between 1991 and 1999, where only the Oxford region had a low EA prevalence of 0.7/10,000 LB. According to the EUROCAT37 data, the only region with a low EA prevalence from 2005 to 2018 similar to that in our study was Southeast Ireland (0.71/10,000 LBs).
The present study found no significant difference of EA proportion in different sex groups, which differs from that in other studies with a higher prevalence in men (M:female (F) sex ratio of 1.3:4.1).1 9 10 38 Although some studies have already shown a higher risk of EA occurrence in white women,1 2 we did not find significant results when considering race/ethnicity as a risk factor.
The results of EA prevalence in different gestational age groups is consistent with those in other literature concerning the relationship between tracheoesophageal anomalies and gestational age, indicating similar findings already described by other authors.2 29–31Even though we are aware of the importance of prenatal consultations,39 we did not observe significant differences regarding the number of consultations carried out in pregnant women with fetuses with EA. Cesarean section is the most frequent type of delivery and has the highest prevalence compared with vaginal delivery, which may be due to the suggestive characteristics of EA found in prenatal ultrasonography, such as polyhydramnios and the association of the absence of a gastric bubble with the dilatation of the cervical esophagus (Pouch sign).40 41 These alterations, when present, often lead obstetricians to opt for cesarean delivery due to the greater possibility of malformation of the digestive tract.
Our results show that EA occurs more frequently in singleton pregnancies but is more prevalent in twin pregnancies. Nazer et al 31 showed similar results in Chile, with EA being two to three times more common in twins; Torfs et al 29 described that twins, especially homozygous twins, were considered a risk factor for the occurrence of EA.
In SPS, we identified a growing prevalence both in the global period (2005–2018) and in the first subperiod (2005–2011). A similar situation occurs throughout the predominantly urban area of the state (SPC and SPMR) and has 58.4% of all identified cases. The other predominantly urban cluster, which presented a slightly different behavior, was BSMR, which also presents an increasing trend in prevalence in the full period, with stationary trends when analyzed by subperiods. These clusters are the most developed and urbanized in the entire state, which leads us to believe that this increase in prevalence is related to better access to diagnostic methods and high-complexity hospitals.29 Remote regions with a lower population density and low birth rate may have less access to highly differentiated resources and thus a lower number of prenatal diagnoses for EA.
The highest EA prevalence and number of EA cases were found in the group with a maternal age of ≥35 years old compared with other maternal age groups. Among adolescent mothers with age of 15–19 years, we found a low number of cases; however, it is the one with the highest APC in the global period, even with a significant LB number decrease from the first to the last year. These results allow us to consider that maternal ages of <20 and ≥35 years are risk factors for the development of EA. Takahashi et al 32 also identified maternal ages of ≤20 and ≥35 years as risk factors for EA. Depaepe et al,30 in Europe, also identified a higher prevalence of EA in mothers aged ≤20 and ≥35 years and a more significant prevalence rate increase in mothers aged 25–29 years. However, Nazer et al 31 did not find significant differences of EA prevalence among the maternal age groups, and the average maternal age was 29.7 years old.
A possible explanation that maternal age of ≥35 years is considered a risk factor for EA occurrence is the susceptibility to fetal pathological alterations, such as congenital malformations and chromosomal alterations more frequently occurring in this age group. In addition, there is also a higher incidence of miscarriages, maternal mortality, gestational diabetes, and pre-eclampsia. Thus, advanced maternal age can be associated with the identification of several birth defects, not just EA.1 29 32 42
We performed a seasonality analysis as described by other authors.1 29 43 Although we did not find a significant monthly seasonal variation based on the conception date over a 7-year study (April 2011– April 2018), the result suggests a trend of higher EA prevalence in the period in which conceptions occurred in the middle of the year, which corresponds to the winter season in the southern hemisphere.
The strengths of this study are the high EA number of cases (n=820) and the 14-year studied period. The main limitation of this study was the presence of incomplete fields or ignored content in the Live Birth Declaration, which was made available by the information system and could be resolved with adequate training of professionals who complete the document or by establishing a dedicated birth defect surveillance system; also, we have no data about fetal deaths. However, we made statistically relevant observations that provide data that can be combined with other future investigations for meta-analyses, both in periods before and after the COVID-19 pandemic.
In conclusion, there is an increasing prevalence trend in SPS, Brazil, from 2005 to 2018 at an average rate of 6.5% per year with no monthly seasonal variation. The increasing prevalence in urban areas indicates improved diagnosis. The lower prevalence from less urbanized areas indicates the need to strengthen diagnostic and treatment facilities in other regions or improve referral systems to facilities with advanced capabilities. The EA risk factors identified are urban area residence, maternal age of <20 and ≥35 years, twins, and prematurity. Although many results of this study were similar to those reported in the literature, there were some differences, mainly regarding the increasing EA prevalence trend.
Data availability statement
Data are available in a public, open access repository. The microdata used for this study is administered by the Live Births Information System (Sistema de Informação Sobre Nascidos Vivos), using data from the Unified Health System Department of Informatics (Departamento de Informática do Sistema Único de Saúde (DATASUS)), maintained by the Ministry of Health of Brazil. DATASUS provides open public access to these data for any purpose (www.datasus.saude.gov.br-http://datasus.saude.gov.br/informacoes–de–saude/tabnet/estatisticas–vitais).
Ethics statements
Patient consent for publication
Ethics approval
This is an audit survey study protocol that uses a secondary, anonymous, public, and open health database and therefore does not need evaluation, authorization, or ethical approval from the Human Research Ethics Committee; nevertheless, we followed the stipulated protocol by national and international standards.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors EFS: conceptualization, data curation and investigation, and writing (original draft). EJFO: data curation and investigation, and writing (original draft). MC: data curation and investigation, and writing (original draft). EFSS: methodology, formal analysis and validation. MGC: conceptualization, methodology, formal analysis and validation, supervision and writing (review and editing). All authors read, reviewed, and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests All authors state that they have no conflicts of interest or competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.