Article Text
Abstract
Background To investigate the accuracy and feasibility of CT in quantification of ventricular volume based on semiautomatic three-dimensional (3D) threshold-based segmentation in porcine heart and children with tetralogy of Fallot (TOF).
Methods Eight porcine hearts were used in the study. The atria were resected and both ventricles of the eight porcine hearts were filled with solidifiable silica gel and performed CT scanning. The water displacement volume of silica gel casting mould was referred as gold standard of ventricular volume. Results of left and right ventricular volumes measured by CT were compared with reference standard. Twenty-three children diagnosed with TOF were retrospectively included. The ventricular volumetric parameters were assessed by cardiac CT before and 6 months after surgery.
Results Left ventricular and right ventricular volumes of porcine hearts measured by CT were highly correlated to casting mould (r=0.845, p=0.008; r=0.933, p=0.001), and there were no statistically significant differences (t=−1.059, p=0.325; t=−1.121, p=0.299). In children with TOF, right ventricular end-systole volumes 6 months after operation were higher than that before surgery, 21.93±4.44 vs 19.80±4.52 mL/m2, p=0.001. Right ventricular ejection fractions 6 months after surgery were lower compared with that before surgery 59.79%±4.26% vs 63.05%±5.04%, p=0.000.
Conclusions CT is able to accurately assess ventricular volumetric parameters based on semiautomatic 3D threshold-based segmentation. Both of the right and left ventricular volumetric parameters could be evaluated by CT in children with TOF.
- cardiac surgery
- congenital abnorm
- imaging
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Cardiac magnetic resonance is regarded as golden standard in assessing cardiac function.
Ventricular volume can be measured by CT based on Simpson method.
Accurate evaluation of right ventricular function and volume is of significance in repaired tetralogy of Fallot (TOF), while two-dimensional (2D) echocardiography is limited in measuring right ventricular function in follow-up for repaired TOF.
What are the new findings?
The accuracy of CT in assessing ventricular volume based on semiautomatic three-dimensional (3D) threshold-based segmentation can be proved by in vitro porcine heart study, which proves that CT is highly accurate.
Both of the left and right ventricular volume in children with repaired TOF can be evaluated by CT based on semiautomatic 3D threshold-based segmentation, which is superior to 2D echocardiography.
How might it impact on clinical practice in the foreseeable future?
The cardiovascular anatomical information and both of the left and right cardiac function of children with repaired TOF can be accurately and quickly assessed by only one CT examination.
Introduction
In patients with congenital heart disease (CHD), ventricular volume and function remain an important issue in predicting the clinical outcomes, especially for children with TOF, since the right ventricular (RV) volume and ejection function (EF) are regarded as a significant index of RV dysfunction.1
Cardiac magnetic resonance (CMR) is currently regarded as a reference standard to assess ventricular volume and function, with the ability to avoid ionising radiation and to quantify flows2–4 compared with CT. However, application of CMR is limited in children, especially in younger infants, due to time-consuming, high expense and need for sedation. In addition, CMR is not applicable to an emergency situation. Echocardiography is mostly used in the first evaluation of congenital heart disease. Considering the RV geometry, assessment of RV volume and function is also challenging in echocardiography. Cardiac CT may be a priority for evaluation of RV function in children with TOF. CT is a widely used non-invasive modality for the assessment of CHD, especially complex CHD, with high spatial resolution, temporal resolution and relatively short examination time.5–9 Considering ionising radiation exposure, CT is commonly used as a complementary tool of echocardiography for evaluation of complex CHD with illustration of intracardiac and extracardiac information.
Recently, CT has allowed the quantification of left ventricular (LV) and RV volume. Several studies demonstrated good correlations between RV functional parameters obtained by CT and CMR10–13 with two-dimensional (2D) manual contouring-based segmentation in CMR and three-dimensional (3D) semiautomatic threshold-based segmentation in CT. CMR 2D manual contouring is relatively accurate, but may take more time than CT 3D semiautomatic threshold-based segmentation. Papillary muscles and trabeculations are included in calculating ventricular volume in 2D CMR segmentation, while they are excluded in CT 3D semiautomatic threshold-based segmentation. Another study showed that LV function parameters measured by CT were similar to CMR, while reducing analysis time.14 In patients with repaired TOF, the quantification of RV function by CT was in good correlation with CMR, but CT may overestimate right ventricular volume (RVV).15 However, in the present study, we attempted to investigate the accuracy of CT in quantification of the left ventricular volume (LVV) and RVV based on semiautomatic 3D threshold-based segmentation using an animal model, which was considered as reference standard, and the feasibility in quantifying ventricular volumetric parameters in children with TOF.
Methods
Porcine heart model
This porcine heart model study aimed to explore the accuracy of CT in quantification of ventricular volume, using a porcine heart model as reference standard. Eight fresh porcine hearts were obtained from local slaughter house and the weight ranged from 420 to 610 g. Left and right atria were resected in order to retain only left and right ventricles, aortic root and pulmonary root. Ventricular cavity was filled with gauze and then fixed in 10% formalin for 10 days. After fixation, both ventricles of porcine heart were filled with silica gel which was able to solidify for left and right ventricles molding. The upper surface of the casting mould was determined by porcine mitral annulus, tricuspid annulus and semilunar annulus. After solidification, these porcine heart models were performed CT scanning. On scanning, the postprocessing was performed to evaluate the volume of left and right ventricles of these porcine hearts, which was based on semiautomatic 3D threshold-based segmentation. At last, all of the porcine hearts models were incised to take out silica gel casting mould. The water displacement of casting mould was referred as the gold standard and compared with ventricular volumes measured by CT.
Study population
Informed consent was waived for this retrospective study. From November 2017 to March 2019, 23 children diagnosed with TOF were retrospectively enrolled in this study. The age ranged from 3.7 to 29.0 months, with an average of 11.8±5.3 months at first CT exam. Their weight ranged from 5.9 to 13.2 kg, with an average of 8.9±2.7 kg at first CT exam. They were performed cardiac CT scanning before surgery and 6 months after corrective surgery. The exclusion criteria were following: patients with coronary disease, pulmonary disease, kidney disease and other birth defects.
CT protocols
Their heart rates ranged from 90 to 130 bpm during scanning with sedation if necessary. All CT examinations were conducted with retrospective ECG-gated, helical acquisition, collimation of 64 detectors×0.625 mm CT scanner (GE Healthcare, USA). The rotation time was 0.35 s, and the pitch was 0.2. Tube voltage was 100 kV. The scanning area ranged from the superior border of the chest to the diaphragm. Field of view was 250 mm×250 mm, matrix size was 512×512 and slice thickness was 0.625 mm. ECG-controlled tube current modulation was applied to reduce radiation dose. All children were injected with 2 mL/kg of non-ionic contrast agent (iopamidol, 370 mg/mL, Bracco, Italy) through a peripheral vein. The injection rate was controlled by an infusion pump within 0.8–2.5 mL/s, according to the size and stability of the intravenous catheter and the patient’s weight. The CT data were exported to the postprocessing workstation (Advantage Windows 4.2, General Electric, Milwaukee, Wisconsin, USA) for advanced evaluation. CT dose index was 1.65±0.15 mGy, dose length product (DLP) was 30.60±8.21 mGy·cm and DLP-based effective dose was 1.46±0.33 mSv.
CT data analysis
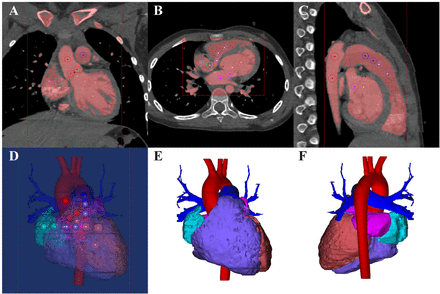
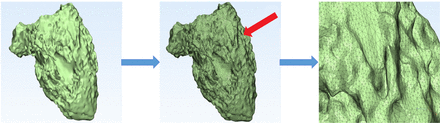
CT image reconstruction was based on retrospective-gated ECG. During one cardiac cycle, the R-R interval was divided into 10% increments and 10 phases were reconstructed. CT data were exported as Digital Imaging and Communications in Medicine and imported into Mimics Innovation Suite 19.0 software (Materialise HQ, Leuven, Belgium) for postprocessing. The end-diastole and end-systole phases were identified visually on the images demonstrating the largest and smallest ventricular and atrial cavity areas, which was determined by a sophisticated radiologist with 20 years’ experience blinded to the clinical information of the patients. The segmentation of the ventricles of heart was semi-automatically performed on Mimics Innovation Suite 19.0 software. For each CT examination, the best threshold for segmentation in each phase was determined to separate the most compact interventricular septal myocardium from the adjacent ventricular blood by radiologists.16 When the related threshold was determined, Mimics software would produce masks covering the included areas. The four chambers of heart, pulmonary artery and aorta were segmented through marking on CT data and at last the 3D volume was obtained as shown in figure 1. The papillary muscles were excluded from ventricular volumes since the segmentation was only based on blood pool. On segmentation, the true volumes of these 3D reconstructed ventricles were automatically measured by Mimics software. The principle of the quantification was based on calculation of voxels. The whole ventricle was automatically divided into large numbers of voxels. And the ventricular volume could be quantified based on the voxels, which is shown in figure 2.
Postprocessing and segmentation of CT data. (A)–(D) With determined threshold, the blood pool was covered by mask. To isolate atria, ventricles, pulmonary artery and aorta, the related areas were tagged by colorful circles on axial, coronary and sagittal planes. (E) and (F) Segmentation of ventricles, atria, pulmonary artery and aorta.
The principle of quantification of volumetric parameters based on semiautomatic three-dimensional threshold-based segmentation.
Statistical analysis
All of the data were presented as means±SDs. The differences between water displacement and CT evaluation were assessed using a paired t-test. The differences in ventricular parameters between preoperation and postoperation were assessed by paired t-test. Pearson’s correlation coefficient was applied to explore the relationship between water displacement and CT evaluation, and a Bland-Altman analysis was used to further determine the agreement between them. In all analyses, a p value of <0.05 was considered statistically significant. All analyses were performed using SPSS V.19.0 software.
Results
Porcine heart models
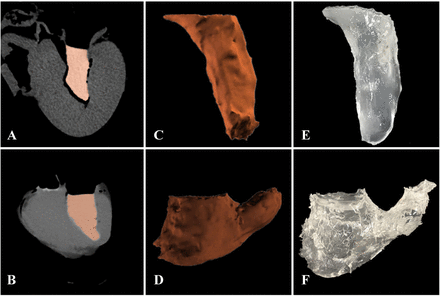
Eight porcine heart models were successfully created and were used as reference standard. CT scanning was performed on all the models. Imaging of solidified silica gel casting mould was in good contrast with myocardium. The border between porcine myocardium and casting mould was extremely clear, making segmentation of casting mould relatively accurate. The average CT attenuation value of the porcine myocardium was 101.3±9.1 HU, while average CT attenuation value of silica gel casting mould was 190.8±10.2 HU. There was a sufficient contrast between them. The geometry of 3D reconstruction was similar to casting mould as shown in figure 3. There was no statistically significant difference between the mean LVV and RVV measured by CT and mean LVV, RVV water displacement (CT vs water displacement: LVV 12.9±1.2 vs 13.2±1.3 mL; p=0.325; RVV 27.7±3.6 vs 28.2±3.2 mL; p=0.299). The Pearson’s correlation coefficients between CT and water displacement quantification of LVV and RVV were r=0.845, p=0.008; r=0.933, p=0.001. Results of Bland-Altman agreement analysis were in good range as shown in figure 4.
The postprocessing and segmentation of CT images of porcine heart models and casting mould. (A) Postprocessing of porcine left ventricle. (B) Postprocessing of porcine right ventricle. (C) Segmentation of porcine left ventricle based on threshold. (D) Segmentation of porcine right ventricle based on threshold. (E) Silica gel casting mould of porcine left ventricle. (F) Silica gel casting mould of porcine right ventricle.
The results of linear regression analysis and Bland-Altman plots between CT measurement and casting mould model water displacement. (A) and (B) LVV. (C) and (D) RVV. The regression equation and Pearson’s correlation coefficient are provided. LVV, left ventricular volume; RVV, right ventricular volume.
CT measurements of ventricular volumes in children with TOF
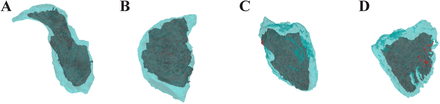
Ventricular volumes of children with TOF could be measured by CT based on semiautomatic 3D threshold-based segmentation. The average of segmentation threshold in TOF was 212.7±20.8 HU, myocardium value was 93.0±10.7 HU, LV cavity value was 204.8±15.6 HU and RV cavity value was 225.9±18.3 HU preoperatively. The average of segmentation threshold was 219.2±23.1 HU, myocardium value was 94.1±9.8 HU, LV cavity value was 205.6±14.5 HU and RV cavity value was 223.8±16.6 HU postoperatively. There was no statistically significant difference between them. The postprocessing costs about 15 min for assessing ventricular volumes. LV, RV and atrial end-diastole and end-systole volumes are illustrated in figure 5. These parameters in children with TOF before and 6 months after surgery are summarised in table 1. The results indicated that 6 months after surgery, right ventricular end-systole volume (RVESV) was higher compared with that before surgery, 21.93±4.44 vs 19.80±4.52 mL/m2, p=0.001. On the other hand, right ventricular ejection fraction (RVEF) decreased 6 months after operation compared with RVEF before surgery, 59.79%±4.26% vs 63.05%±5.04%, p=0.000.
The segmentation and postprocessing results of (A) left atrium, (B) right atrium, (C) left ventricle, (D) right ventricle. Based on semiautomatic three-dimensional threshold-based segmentation at end-systole (dark color) and end-diastole (light color).
Volumetric parameters in children with repaired and unrepaired TOF assessed by CT
Discussion
Quantification of ventricular volume and function is of great importance in predicting outcomes of CHD.17 In patients with repaired TOF, RVV and function are very significant markers in predicting the long-term outcomes.18 However, how to evaluate the RV function of patients with TOF has always been a difficult problem to be solved clinically. Therefore, accurate measurement of RVV and LVV function in TOF and other CHD remains an important issue. This study is an attempt to further report the CT quantification of ventricular volume based on semiautomatic 3D threshold-based segmentation in children with unrepaired TOF and follow-up as previously reported and is a valuable validation study of CT volumetric evaluation using porcine models. The results of this preliminary research demonstrated the feasibility and accuracy of CT in assessing ventricular volumetric parameters.
CMR is identified as the reference standard in measuring ventricular volume and function. Due to the limitation of the retrospective nature of this study on children, to explore the accuracy of CT in quantifying ventricular volumes, a porcine heart model with silica gel casting mould was used as a reference standard instead of CMR. In previous in vitro studies, animal model was considered the gold standard of ventricular volume.19–21 A previous in vivo animal study demonstrated that CT could accurately determine LV volumes in comparison to conventional ventriculography.22 The results of animal study demonstrated CT measurement of porcine ventricular volume was highly accurate. The differences between CT evaluation and water displacement of casting mould were extremely small. The LVV and RVV between CT measurement and true volume in our in vitro study was highly correlated (LVV r=0.845, p=0.008; RVV r=0.933, p=0.001), suggesting that CT is a promising accurate modality in the assessment of ventricular volume.
The results of this study demonstrated that 6 months after TOF surgery, left ventricular ejection fraction (LVEF) remained unchanged compared with LVEF before surgery. However, in repaired TOF, LVEF and LV preload will get normal when the malformation is corrected. But right ventricular end-diastole volume and RVESV increased 6 months after surgery. Particularly in RVESV, there was a significant statistical difference compared with that before surgery. And this may due to the increased RV volume load caused by pulmonary regurgitation after TOF radical surgery. The volume of the right ventricle increases significantly with the increase in pulmonary regurgitation after TOF correction, although the right ventricle is well tolerated by chronic volume overload, due to chronic free wall expansion caused by chronic volume overload of the right ventricle and ventricular septal dysfunction caused by ventricular septal shift to the LV side, thereby leading to a chronic decline in RV systolic function.23 When the RV systolic function drops to <45%, the patient develops clinical symptoms.24 In this study, although patients with TOF had no clinical symptoms at 6 months after surgery, their RVEF was significantly lower compared with that before surgery, suggesting the decline of RV systolic function in patients with TOF was earlier than the appearance of clinical symptoms. Therefore, CT can be used to detect the RV systolic function in patients with TOF after early follow-up of TOF, so that intervention can be taken as soon as possible to improve the RV systolic function and the long-term quality of life after surgery and life expectancy. The use of CT to assess postoperative RV systolic function in patients with TOF is feasible in postoperative follow-up of TOF.
Currently, the segmentation method in CMR is a simplified 2D endocardial contouring using short-axis or axial cine MRI, which is identified as reference standard. However, the inclusion and exclusion of papillary muscles and trabeculations as a part of ventricular volume may result in different results,25–29 leading to overestimation of ventricular volume and underestimation of EF. Otherwise, the simplified endocardial contouring is also subject to the operator variability. In our study, the papillary muscles and trabeculations were excluded. The blood pool, excluding papillary muscles and trabeculations, is the true volume of ventricle.
There are several limitations in our study. First, this is a retrospective study and the number of patients is relatively small. In the future, a larger number of patients should be enrolled. However, exposure of ionising radiation is inevitable with CT. Second, CMR assessment of ventricular volume and function in children could not be used as reference standard, because they had only undergone CT examinations. As a result, an animal study was designed to evaluate the accuracy of CT measurement of ventricular volume. Third, the accuracy of quantification of ventricular volume largely depends on the quality of CT images. The volume of blood pool, identified as the true volumetric parameter, is commonly influenced by the uniformity of contrast medium. The unsatisfied uniformity of contrast medium will result in underestimation of ventricular volume, which can be technically resolved.
Conclusions
CT is able to accurately assess ventricular volumetric parameters and functions based on semiautomatic 3D threshold-based segmentation. In evaluation of unrepaired TOF and follow-up of children with repaired TOF, CT could assess both the anatomical information and volumetric parameters.
References
Footnotes
Contributors Conception and design: JX, JW. Administrative support: QS, JF. Provision of study materials or patients: JW, WX, ZS. Collection and assembly of data: JX. Data analysis and interpretation: JX. Manuscript writing: all authors. Final approval of manuscript: all authors.
Funding This study was funded by Science Technology Department of Zhejiang Province of China (grant number: 2016C54006).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.