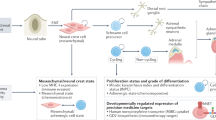
Key Points
-
Neuroblastomas in younger children have a higher propensity to undergo spontaneous regression than any other human malignancy, but only genetic subtype 1 tumours with numerical chromosome aberrations are prone to spontaneous regression
-
Although spontaneous regression of neuroblastoma is strongly associated with stage 4S disease, mass-screening studies suggest that genetic subtype 1 tumours of any stage in infants <18 months can undergo spontaneous regression
-
The TrkA/NGF pathway might have a major role in causing spontaneous regression, but alternative mechanisms might involve cellular or humoral immune responses, telomere shortening, or epigenetic modifications
-
Inhibition of the TrkA pathway represents the most-promising approach to initiate regression in infants with favourable tumours; other approaches involving immune modulation or epigenetic regulation are being investigated
Abstract
Recent genomic and biological studies of neuroblastoma have shed light on the dramatic heterogeneity in the clinical behaviour of this disease, which spans from spontaneous regression or differentiation in some patients, to relentless disease progression in others, despite intensive multimodality therapy. This evidence also suggests several possible mechanisms to explain the phenomena of spontaneous regression in neuroblastomas, including neurotrophin deprivation, humoral or cellular immunity, loss of telomerase activity and alterations in epigenetic regulation. A better understanding of the mechanisms of spontaneous regression might help to identify optimal therapeutic approaches for patients with these tumours. Currently, the most druggable mechanism is the delayed activation of developmentally programmed cell death regulated by the tropomyosin receptor kinase A pathway. Indeed, targeted therapy aimed at inhibiting neurotrophin receptors might be used in lieu of conventional chemotherapy or radiation in infants with biologically favourable tumours that require treatment. Alternative approaches consist of breaking immune tolerance to tumour antigens or activating neurotrophin receptor pathways to induce neuronal differentiation. These approaches are likely to be most effective against biologically favourable tumours, but they might also provide insights into treatment of biologically unfavourable tumours. We describe the different mechanisms of spontaneous neuroblastoma regression and the consequent therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brodeur, G. M. & Maris, J. M. in Principles and Practice of Pediatric Oncology (eds Pizzo, P. A. & Poplack, D. G.) 786–822 (Lippincott, Williams and Wilkins, Philadelphia, 2010).
Maris, J. M., Hogarty, M. D., Bagatell, R. & Cohn, S. L. Neuroblastoma. Lancet 369, 2106–2120 (2007).
Smith, M. A. et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J. Clin. Oncol. 28, 2625–2634 (2010).
Gatta, G. et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 15, 35–47 (2014).
Kreissman, S. G. et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 14, 999–1008 (2013).
Diede, S. J. Spontaneous regression of metastatic cancer: learning from neuroblastoma. Nat. Rev. Cancer 14, 71–72 (2014).
Matthay, K. K. Stage 4S neuroblastoma: what makes it special? J. Clin. Oncol. 16, 2003–2006 (1998).
Nakagawara, A. Molecular basis of spontaneous regression of neuroblastoma: role of neurotrophic signals and genetic abnormalities. Hum. Cell 11, 115–124 (1998).
Nickerson, H. J. et al. Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: a Children's Cancer Group study. J. Clin. Oncol. 18, 477–486 (2000).
Pritchard, J. & Hickman, J. A. Why does stage 4s neuroblastoma regress spontaneously? Lancet 344, 869–870 (1994).
Yamamoto, K. et al. Marginal decrease in mortality and marked increase in incidence as a result of neuroblastoma screening at 6 months of age: cohort study in seven prefectures in Japan. J. Clin. Oncol. 20, 1209–1214 (2002).
Sawada, T. et al. Mass screening for neuroblastoma in Japan. Pediatr. Hematol. Oncol. 8, 93–109 (1991).
Erttmann, R. et al. 10 years' neuroblastoma screening in Europe: preliminary results of a clinical and biological review from the Study Group for Evaluation of Neuroblastoma Screening in Europe (SENSE). Eur. J. Cancer 34, 1391–1397 (1998).
Woods, W. G. et al. A population-based study of the usefulness of screening for neuroblastoma. Lancet 348, 1682–1687 (1996).
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216 (2003).
Hoehner, J. C., Olsen, L., Sandstedt, B., Kaplan, D. R. & Pahlman, S. Association of neurotrophin receptor expression and differentiation in human neuroblastoma. Am. J. Pathol. 147, 102–113 (1995).
Haas, D., Ablin, A. R., Miller, C., Zoger, S. & Matthay, K. K. Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer 62, 818–825 (1988).
Garvin, J. H. Jr, Lack, E. E., Berenberg, W. & Frantz, C. N. Ganglioneuroma presenting with differentiated skeletal metastases. Report of a case. Cancer 54, 357–360 (1984).
Shimada, H. et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 86, 349–363 (1999).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Shojaei-Brosseau, T. et al. Genetic epidemiology of neuroblastoma: a study of 426 cases at the Institut Gustave-Roussy in France. Pediatr. Blood Cancer 42, 99–105 (2004).
Mosse, Y. P. et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am. J. Hum. Genet. 75, 727–730 (2004).
Maris, J. M. et al. Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12–13 Cancer Res. 62, 6651–6658 (2002).
Chen, Y. et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455, 971–974 (2008).
George, R. E. et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455, 975–978 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Raabe, E. H. et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene 27, 469–476 (2008).
Trochet, D. et al. Germline mutations of the paired-like homeoBox 2B (PHOX2B) gene in neuroblastoma. Am. J. Hum. Genet. 74, 761–764 (2004).
Bosse, K. R. et al. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res. 72, 2068–2078 (2012).
Capasso, M. et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat. Genet. 41, 718–723 (2009).
Diskin, S. J. et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat. Genet. 44, 1126–1130 (2012).
Nguyen le, B. et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 7, e1002026 (2011).
Wang, K. et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature 469, 216–220 (2011).
Weiss, W. A., Aldape, K., Mohapatra, G., Feuerstein, B. G. & Bishop, J. M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16, 2985–2995 (1997).
Chen, Z. et al. Mdm2 deficiency suppresses MYCN-driven neuroblastoma tumorigenesis in vivo. Neoplasia 11, 753–762 (2009).
Berry, T. et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 22, 117–130 (2012).
Heukamp, L. C. et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Sci. Transl. Med. 4, 141ra91 (2012).
Molenaar, J. J. et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet. 44, 1199–1206 (2012).
Mosse, Y. P. et al. Neuroblastomas have distinct genomic DNA profiles that predict clinical phenotype and regional gene expression. Genes Chromosomes Cancer 46, 936–949 (2007).
Schleiermacher, G. et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br. J. Cancer 107, 1418–1422 (2012).
Pugh, T. J. et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 45, 279–284 (2013).
Cheung, N. K. et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 307, 1062–1071 (2012).
Sausen, M. et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat. Genet. 45, 12–17 (2013).
Molenaar, J. J. et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593 (2012).
Santo, E. E. et al. Oncogenic activation of FOXR1 by 11q23 intrachromosomal deletion-fusions in neuroblastoma. Oncogene 31, 1571–1581 (2012).
Benard, J. et al. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol. Oncol. 2, 261–271 (2008).
Diskin, S. J. et al. Integrative genomic and epigenomic characterization of stage 4S neuroblastoma gene expression [abstract]. Advances in Neuroblastoma Research, POB083 (Cologne, 2014).
Taggart, D. R. et al. Prognostic value of the stage 4S metastatic pattern and tumor biology in patients with metastatic neuroblastoma diagnosed between birth and 18 months of age. J. Clin. Oncol. 29, 4358–4364 (2011).
Challis, G. B. & Stam, H. J. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 29, 545–550 (1990).
Everson, T. C. Spontaneous regression of cancer. Ann. N.Y. Acad. Sci. 114, 721–735 (1964).
Everson, T. C. & Cole, W. H. Spontaneous regression of cancer (W. B. Saunders & Co., Philadelphia, 1966).
Papac, R. J. Spontaneous regression of cancer: possible mechanisms. In Vivo 12, 571–578 (1998).
Beckwith, J. B. & Perrin, E. V. In situ neuroblastomas: a contribution to the natural history of neural crest tumors. Am. J. Pathol. 43, 1089–1104 (1963).
Ikeda, Y., Lister, J., Bouton, J. M. & Buyukpamukcu, M. Congenital neuroblastoma, neuroblastoma in situ, and the normal fetal development of the adrenal. J. Pediatr. Surg. 16, 636–644 (1981).
Turkel, S. B. & Itabashi, H. H. The natural history of neuroblastic cells in the fetal adrenal gland. Am. J. Pathol. 76, 225–244 (1974).
D'Angio, G. J., Evans, A. E. & Koop, C. E. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet 1, 1046–1049 (1971).
Evans, A. E., D'Angio, G. J. & Randolph, J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer 27, 374–378 (1971).
George, R. E. et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J. Clin. Oncol. 24, 2891–2896 (2006).
Matthay, K. K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N. Engl. J. Med. 341, 1165–1173 (1999).
Brodeur, G. M. et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 11, 1466–1477 (1993).
Brodeur, G. M. et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J. Clin. Oncol. 6, 1874–1881 (1988).
Monclair, T. et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J. Clin. Oncol. 27, 298–303 (2009).
Cozzi, D. A. et al. Long-term follow-up of the “wait and see” approach to localized perinatal adrenal neuroblastoma. World J. Surg. 37, 459–465 (2013).
Fisher, J. P. & Tweddle, D. A. Neonatal neuroblastoma. Semin. Fetal Neonatal Med. 17, 207–215 (2012).
Kushner, B. H. et al. Survival from locally invasive or widespread neuroblastoma without cytotoxic therapy. J. Clin. Oncol. 14, 373–381 (1996).
Lavarino, C. et al. Specific gene expression profiles and chromosomal abnormalities are associated with infant disseminated neuroblastoma. BMC Cancer 9, 44 (2009).
Yu, F. et al. Proteomics-based identification of spontaneous regression-associated proteins in neuroblastoma. J. Pediatr. Surg. 46, 1948–1955 (2011).
Knudson, A. G. Jr & Meadows, A. T. Regression of neuroblastoma IV-S: a genetic hypothesis. N. Engl. J. Med. 302, 1254–1256 (1980).
van Noesel, M. M. Neuroblastoma stage 4S: a multifocal stem-cell disease of the developing neural crest. Lancet Oncol. 13, 229–230 (2012).
Spitz, R. et al. Favorable outcome of triploid neuroblastomas: a contribution to the special oncogenesis of neuroblastoma. Cancer Genet. Cytogenet. 167, 51–56 (2006).
Ambros, P. F. et al. Regression and progression in neuroblastoma. Does genetics predict tumour behaviour? Eur. J. Cancer 31A, 510–515 (1995).
LaBrosse, E. H., Comoy, E., Bohuon, C., Zucker, J. M. & Schweisguth, O. Catecholamine metabolism in neuroblastoma. J. Natl Cancer Inst. 57, 633–638 (1976).
Bessho, F. Comparison of the incidences of neuroblastoma for screened and unscreened cohorts. Acta Paediatr. 88, 404–406 (1999).
Schilling, F. H. et al. Neuroblastoma screening at one year of age. N. Engl. J. Med. 346, 1047–1053 (2002).
Woods, W. G. et al. Screening of infants and mortality due to neuroblastoma. N. Engl. J. Med. 346, 1041–1046 (2002).
Yamamoto, K. et al. Marginal decrease in mortality and marked increase in incidence as a result of neuroblastoma screening at 6 months of age: cohort study in seven prefectures in Japan. J. Clin. Oncol. 20, 1209–1214 (2002).
Brodeur, G. M., Ambros, P. F. & Favrot, M. C. Biological aspects of neuroblastoma screening. Med. Ped. Oncol. 31, 394–400 (1998).
Hayashi, Y., Hanada, R. & Yamamoto, K. Biology of neuroblastomas in Japan found by screening. Am. J. Pediatr. Hematol. Oncol. 14, 342–347 (1992).
Kaneko, Y. et al. Current urinary mass screening for catecholamine metabolites at 6 months of age may be detecting only a small portion of high-risk neuroblastomas: A chromosome and N-myc amplification study. J. Clin. Oncol. 8, 2005–2013 (1990).
Acharya, S. et al. Prenatally diagnosed neuroblastoma. Cancer 80, 304–310 (1997).
Ho, P. T. et al. Prenatal detection of neuroblastoma: a ten-year experience from the Dana-Farber Cancer Institute and Children's Hospital. Pediatrics 92, 358–364 (1993).
Saylors, R. L. 3rd, Cohn, S. L., Morgan, E. R. & Brodeur, G. M. Prenatal detection of neuroblastoma by fetal ultrasonography. Am. J. Pediatr. Hematol. Oncol. 16, 356–360 (1994).
Ikeda, H. et al. Surgical treatment of neuroblastomas in infants under 12 months of age. J. Pediatr. Surg. 33, 1246–1250 (1998).
Hero, B. et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J. Clin. Oncol. 26, 1504–1510 (2008).
Oue, T. et al. Profile of neuroblastoma detected by mass screening, resected after observation without treatment: results of the Wait and See pilot study. J. Pediatr. Surg. 40, 359–363 (2005).
Nishihira, H. et al. Natural course of neuroblastoma detected by mass screening: a 5-year prospective study at a single institution. J. Clin. Oncol. 18, 3012–3017 (2000).
Nuchtern, J. G. et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children's Oncology Group study. Ann. Surg. 256, 573–580 (2012).
Brodeur, G. M. et al. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 15, 3244–3250 (2009).
Brodeur, G. M. et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J. Neurooncol. 31, 49–55 (1997).
Thiele, C. J., Li, Z. & McKee, A. E. On Trk—the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin. Cancer Res. 15, 5962–5967 (2009).
Kogner, P. et al. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 53, 2044–2050 (1993).
Nakagawara, A., Arima, M., Azar, C. G., Scavarda, N. J. & Brodeur, G. M. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 52, 1364–1368 (1992).
Nakagawara, A. et al. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N. Engl. J. Med. 328, 847–854 (1993).
Stram, D. & Seeger, R. C. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J. Natl Cancer Inst. 85, 377–384 (1993).
Nakagawara, A., Azar, C. G., Scavarda, N. J. & Brodeur, G. M. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol. Cell Biol. 14, 759–767 (1994).
Acheson, A. et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374, 450–453 (1995).
Jaboin, J., Kim, C. J., Kaplan, D. R. & Thiele, C. J. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 62, 6756–6763 (2002).
Matsumoto, K., Wada, R. K., Yamashiro, J. M., Kaplan, D. R. & Thiele, C. J. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 55, 1798–1806 (1995).
Nakamura, K. et al. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 66, 4249–4255 (2006).
Goldschneider, D. & Mehlen, P. Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 29, 1865–1882 (2010).
Rabizadeh, S., Ye, X., Wang, J. J. & Bredesen, D. E. Neurotrophin dependence mediated by p75NTR: contrast between rescue by BDNF and NGF. Cell Death Differ. 6, 1222–1227 (1999).
Hantzopoulos, P. A., Suri, C., Glass, D. J., Goldfarb, M. P. & Yancopoulos, G. D. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron 13, 187–201 (1994).
Ho, R. et al. The effect of P75 on Trk receptors in neuroblastomas. Cancer Lett. 305, 76–85 (2011).
Bamji, S. X. et al. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J. Cell Biol. 140, 911–923 (1998).
Nakagawara, A. & Brodeur, G. M. Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur. J. Cancer 33, 2050–2053 (1997).
Tacconelli, A. et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 6, 347–360 (2004).
Tacconelli, A., Farina, A. R., Cappabianca, L., Gulino, A. & Mackay, A. R. Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Oncol. 1, 689–698 (2005).
Kahane, N. & Kalcheim, C. Expression of trkC receptor mRNA during development of the avian nervous system. J. Neurobiol. 25, 571–584 (1994).
Pachnis, V., Mankoo, B. & Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, 1005–1017 (1993).
Tsuzuki, T. et al. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene 10, 191–198 (1995).
Salcedo, R. et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J. Immunol. 182, 4328–4338 (2009).
Salcedo, R. et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J. Immunol. 173, 7170–7182 (2004).
Antunes, N. L. et al. Antineuronal antibodies in patients with neuroblastoma and paraneoplastic opsoclonus-myoclonus. J. Pediatr. Hematol. Oncol. 22, 315–320 (2000).
Kataoka, Y., Matsumura, T., Yamamoto, S., Sugimoto, T. & Sawada, T. Distinct cytotoxicity against neuroblastoma cells of peripheral blood and tumor-infiltrating lymphocytes from patients with neuroblastoma. Cancer Lett. 73, 11–21 (1993).
Valteau, D. et al. T-cell receptor repertoire in neuroblastoma patients. Cancer Res. 56, 362–369 (1996).
Cooper, R. et al. Opsoclonus-myoclonus-ataxia syndrome in neuroblastoma: histopathologic features-a report from the Children's Cancer Group. Med. Pediatr. Oncol. 36, 623–629 (2001).
Pranzatelli, M. R. et al. B- and T-cell markers in opsoclonus-myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology 62, 1526–1532 (2004).
Rudnick, E. et al. Opsoclonus-myoclonus-ataxia syndrome in neuroblastoma: clinical outcome and antineuronal antibodies-a report from the Children's Cancer Group Study. Med. Pediatr. Oncol. 36, 612–622 (2001).
Russo, C., Cohn, S. L., Petruzzi, M. J. & de Alarcon, P. A. Long-term neurologic outcome in children with opsoclonus-myoclonus associated with neuroblastoma: a report from the Pediatric Oncology Group. Med. Pediatr. Oncol. 28, 284–288 (1997).
Raffaghello, L. et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene 24, 4634–4644 (2005).
Squire, R., Fowler, C. L., Brooks, S. P., Rich, G. A. & Cooney, D. R. The relationship of class I MHC antigen expression to stage IV-S disease and survival in neuroblastoma. J. Pediatr. Surg. 25, 381–386 (1990).
Bin, Q., Johnson, B. D., Schauer, D. W., Casper, J. T. & Orentas, R. J. Production of macrophage migration inhibitory factor by human and murine neuroblastoma. Tumour Biol. 23, 123–129 (2002).
Castriconi, R. et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 64, 9180–9184 (2004).
Raffaghello, L. et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 6, 558–568 (2004).
Ren, Y. et al. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene 25, 3501–3508 (2006).
Asgharzadeh, S. et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J. Clin. Oncol. 30, 3525–3532 (2012).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Hiyama, E. et al. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat. Med. 1, 249–255 (1995).
Samy, M. et al. Loss of the malignant phenotype of human neuroblastoma cells by a catalytically inactive dominant-negative hTERT mutant. Mol. Cancer Ther. 11, 2384–2393 (2012).
Brodeur, G. M. Do the ends justify the means? Nat. Med. 1, 203–205 (1995).
Krams, M. et al. Full-length telomerase reverse transcriptase messenger RNA is an independent prognostic factor in neuroblastoma. Am. J. Pathol. 162, 1019–1026 (2003).
Ohali, A. et al. Telomere length is a prognostic factor in neuroblastoma. Cancer 107, 1391–1399 (2006).
Streutker, C. J., Thorner, P., Fabricius, N., Weitzman, S. & Zielenska, M. Telomerase activity as a prognostic factor in neuroblastomas. Pediatr. Dev. Pathol. 4, 62–67 (2001).
Astuti, D. et al. RASSF1A promoter region CpG island hypermethylation in phaeochromocytomas and neuroblastoma tumours. Oncogene 20, 7573–7577 (2001).
Takita, J. et al. Absent or reduced expression of the caspase 8 gene occurs frequently in neuroblastoma, but not commonly in Ewing sarcoma or rhabdomyosarcoma. Med. Pediatr. Oncol. 35, 541–543 (2000).
Barbieri, E. et al. Histone chaperone CHAF1A inhibits differentiation and promotes aggressive neuroblastoma. Cancer Res. 74, 765–774 (2014).
Grau, E. et al. Epigenetic alterations in disseminated neuroblastoma tumour cells: influence of TMS1 gene hypermethylation in relapse risk in NB patients. J. Cancer Res. Clin. Oncol. 136, 1415–1421 (2010).
Yang, Q. et al. Methylation of CASP8, DCR2, and HIN-1 in neuroblastoma is associated with poor outcome. Clin. Cancer Res. 13, 3191–3197 (2007).
Decock, A., Ongenaert, M., Vandesompele, J. & Speleman, F. Neuroblastoma epigenetics: from candidate gene approaches to genome-wide screenings. Epigenetics 6, 962–970 (2011).
Batora, N. V. et al. Transitioning from genotypes to epigenotypes: why the time has come for medulloblastoma epigenomics. Neuroscience 264, 171–185 (2014).
Feinberg, A. P. The epigenetics of cancer etiology. Semin. Cancer Biol. 14, 427–432 (2004).
Feinberg, A. P. & Tycko, B. The history of cancer epigenetics. Nat. Rev. Cancer 4, 143–153 (2004).
Baylin, S. B. DNA methylation and gene silencing in cancer. Nat. Clin. Pract. Oncol. 2 (Suppl. 1), S4–S11 (2005).
Gros, C. et al. DNA methylation inhibitors in cancer: recent and future approaches. Biochimie 94, 2280–2296 (2012).
McCabe, M. T. & Creasy, C. L. EZH2 as a potential target in cancer therapy. Epigenomics 6, 341–351 (2014).
Evans, A. E. et al. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med. Ped. Oncol. 36, 181–184 (2001).
Evans, A. E. et al. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin. Cancer Res. 5, 3594–3602 (1999).
Ho, R. et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 62, 6462–6466 (2002).
Iyer, R. et al. Lestaurtinib enhances the antitumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin. Cancer Res. 16, 1478–1485 (2010).
Minturn, J. E. et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother. Pharmacol. 68, 1057–1065 (2011).
De Braud, F. G. et al. Phase 1 open label, dose escalation study of RXDX101, an oral pan-trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations [abstract]. J. Clin. Oncol. 32 (Suppl.), a2502 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine.ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Hsu, L. L., Evans, A. E. & D'Angio, G. J. Hepatomegaly in neuroblastoma stage 4s: criteria for treatment of the vulnerable neonate. Med. Pediatr. Oncol. 27, 521–528 (1996).
Kushner, B. H., Kramer, K., LaQuaglia, M. P., Modak, S. & Cheung, N. K. Liver involvement in neuroblastoma: the Memorial Sloan-Kettering Experience supports treatment reduction in young patients. Pediatr. Blood Cancer 46, 278–284 (2006).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Baker, D. L. et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N. Engl. J. Med. 363, 1313–1323 (2010).
Yuza, Y., Agawa, M., Matsuzaki, M., Yamada, H. & Urashima, M. Gene and protein expression profiling during differentiation of neuroblastoma cells triggered by 13-cis retinoic acid. J. Pediatr. Hematol. Oncol. 25, 715–720 (2003).
Shimada, H. et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86, 364–372 (1999).
Fouladi, M. et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J. Clin. Oncol. 28, 3623–3629 (2010).
Park, J. R. et al. A phase I study of vorinostat in combination with isotretinoin (RA) in patients with refractory/recurrent neuroblastoma (NB): a new approaches to neuroblastoma therapy consortium trial [abstract]. Advances in Neuroblastoma Research, OR070 (Cologne, 2014).
Simoes-Costa, M. & Bronner, M. E. Insights into neural crest development and evolution from genomic analysis. Genome Res. 23, 1069–1080 (2013).
Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu. Rev. Cell. Dev. Biol. 26, 581–603 (2010).
De Preter, K. et al. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 7, R84 (2006).
Tsarovina, K. et al. Essential role of GATA transcription factors in sympathetic neuron development. Development 131, 4775–4786 (2004).
Unsicker, K., Huber, K., Schober, A. & Kalcheim, C. Resolved and open issues in chromaffin cell development. Mech. Dev. 130, 324–329 (2013).
Pei, D. et al. Distinct neuroblastoma-associated alterations of PHOX2B impair sympathetic neuronal differentiation in zebrafish models. PLoS Genet. 9, e1003533 (2013).
Shimada, H. et al. Histopathologic prognostic factors in neuroblastic tumors: Definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J. Natl Cancer Inst. 73, 405–413 (1984).
Shimada, H. et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children's Cancer Group. Cancer 92, 2451–2461 (2001).
Ambros, I. M. et al. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N. Engl. J. Med. 334, 1505–1511 (1996).
Brodeur, G. M. Schwann cells as antineuroblastoma agents. N. Engl. J. Med. 334, 1537–1539 (1996).
Acknowledgements
This work was supported in part by NIH grants R01-CA094194 and R01-039,771; Alex's Lemonade Stand Foundation; and the Audrey E. Evans endowed chair (G.M.B.).
Author information
Authors and Affiliations
Contributions
G.M.B. and R.B. researched data and discussed content for article, wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brodeur, G., Bagatell, R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol 11, 704–713 (2014). https://doi.org/10.1038/nrclinonc.2014.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.168
This article is cited by
-
p53 stabilisation potentiates [177Lu]Lu-DOTATATE treatment in neuroblastoma xenografts
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Perioperative complication incidence and risk factors for retroperitoneal neuroblastoma in children: analysis of 571 patients
World Journal of Pediatrics (2024)
-
Serum brain-derived neurotrophic factor (BDNF) as predictors of childhood neuroblastoma relapse
BMC Cancer (2023)
-
The immune landscape of solid pediatric tumors
Journal of Experimental & Clinical Cancer Research (2022)
-
Neuroblastoma Heterogeneity, Plasticity, and Emerging Therapies
Current Oncology Reports (2022)