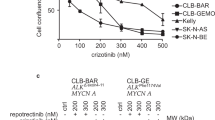
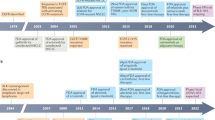
Abstract
Despite improvements in cancer therapies in the past 50 years, neuroblastoma remains a devastating clinical problem and a leading cause of childhood cancer deaths. Advances in treatments for children with high-risk neuroblastoma have, until recently, involved addition of cytotoxic therapy to dose-intensive regimens. In this era of targeted therapies, substantial efforts have been made to identify optimal targets for different types of cancer. The discovery of hereditary and somatic activating mutations in the oncogene ALK has now placed neuroblastoma among other cancers, such as melanoma and non-small-cell lung cancer (NSCLC), which benefit from therapies with oncogene-specific small-molecule tyrosine kinase inhibitors. Crizotinib, a small-molecule inhibitor of ALK, has transformed the landscape for the treatment of NSCLC harbouring ALK translocations and has demonstrated activity in preclinical models of ALK-driven neuroblastomas. However, inhibition of mutated ALK is complex when compared with translocated ALK and remains a therapeutic challenge. This Review discusses the biology of ALK in the development of neuroblastoma, preclinical and clinical progress with the use of ALK inhibitors and immunotherapy, challenges associated with resistance to such therapies and the steps being taken to overcome some of these hurdles.
Key Points
-
The discovery of germline and somatic mutations in ALK provides the first tractable oncogenic target in neuroblastoma, prompting the initiation of a phase I–II trial of the ALK inhibitor crizotinib
-
ALK is mutated in 8% of all neuroblastoma cases; mutations are distributed across the range of phenotypes and predict for an inferior outcome
-
Full-length ALK is expressed on the surface of neuroblastoma tumours in both the presence and absence of a genetic alteration, suggesting that ALK antibody therapy is relevant in neuroblastoma
-
The F1174L mutation in ALK results in de novo resistance in neuroblastoma and has emerged as a resistance mechanism in ALK-translocated tumours treated with crizotinib
-
Structural and biochemical data suggest that the increase in ATP-binding affinity for the F1174L-mutated ALK can be overcome with higher doses of crizotinib
-
Stratification of patients based on ALK alteration status will likely become an integral part of frontline therapy for patients with high-risk neuroblastoma
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Smith, M. A. et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J. Clin. Oncol. 28, 2625–2634 (2010).
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216 (2003).
Maris, J. M. Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211 (2010).
Carlsen, N. L. How frequent is spontaneous remission of neuroblastomas? Implications for screening. Br. J. Cancer 61, 441–446 (1990).
Everson, T. C. & Cole, W. H. Spontaneous regression of neuroblastoma. 88–163 (W. B. Saunders Co., Philadelphia, 1966).
Yamamoto, K. et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J. Clin. Oncol. 16, 1265–1269 (1998).
Attiyeh, E. F. et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N. Engl. J. Med. 353, 2243–2253 (2005).
Brodeur, G., Seeger, R. C., Schwab, M., Varmus, H. E. & Bishop, J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984).
Schwab, M. et al. Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma. Proc. Natl Acad. Sci. USA 81, 4940–4944 (1984).
Matthay, K. K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N. Engl. J. Med. 341, 1165–1173 (1999).
Sidell, N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J. Natl Cancer Inst. 68, 589–596 (1982).
Thiele, C. J., Reynolds, C. P. & Israel, M. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature 313, 404–406 (1986).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Brodeur, G. M. et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J. Neurooncol. 31, 49–55 (1997).
Minturn, J. E. et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother. Pharmacol. 68, 1057–1065 (2011).
Norris, R. E., Minturn, J. E., Brodeur, G. M., Maris, J. M. & Adamson, P. C. Preclinical evaluation of lestaurtinib (CEP-701) in combination with retinoids for neuroblastoma. Cancer Chemother. Pharmacol. 68, 1469–1475 (2011).
Beppu, K., Jaboine, J., Merchant, M. S., Mackall, C. L. & Thiele, C. J. Effect of imatinib mesylate on neuroblastoma tumorigenesis and vascular endothelial growth factor expression. J. Natl Cancer Inst. 96, 46–55 (2004).
Vitali, R. et al. c-Kit is preferentially expressed in MYCN-amplified neuroblastoma and its effect on cell proliferation is inhibited in vitro by STI-571. Int. J. Cancer 106, 147–152 (2003).
Eggert, A. et al. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 6, 1900–1908 (2000).
Fotsis, T. et al. Down-regulation of endothelial cell growth inhibitors by enhanced MYCN oncogene expression in human neuroblastoma cells. Eur. J. Biochem. 263, 757–764 (1999).
Maris, J. M. et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr. Blood Cancer 50, 581–587 (2008).
Coffey, D. C. et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 61, 3591–3594 (2001).
Jaboin, J. et al. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 62, 6108–6115 (2002).
Goldsmith, K. C. & Hogarty, M.D. Targeting programmed cell death pathways with experimental therapeutics: opportunities in high-risk neuroblastoma. Cancer Lett. 228, 133–141 (2005).
Osajima-Hakomori, Y. et al. Biological role of anaplastic lymphoma kinase in neuroblastoma. Am. J. Pathol. 167, 213–222 (2005).
Lim, M. S. et al. The proteomic signature of NPM/ALK reveals deregulation of multiple cellular pathways. Blood 114, 1585–1595 (2009).
Palmer, R. H., Vernersson, E., Grabbe, C. & Hallberg, B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem. J. 420, 345–361 (2009).
Carpenter, E. L. et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene http://dx.doi.org/10.1038/onc.2011.647.
Iwahara, T. et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14, 439–449 (1997).
Morris, S. W. et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263, 1281–1284 (1994).
Cheng, M. & Ott, G. R. Anaplastic lymphoma kinase as a therapeutic target in anaplastic large cell lymphoma, non-small cell lung cancer and neuroblastoma. Anticancer Agents Med. Chem. 10, 236–249 (2010).
Sugawara, E. et al. Identification of anaplastic lymphoma kinase fusions in renal cancer: Large-scale immunohistochemical screening by the intercalated antibody-enhanced polymer method. Cancer http://dx.doi.org/10.1002/cncr.27391.
Mosse, Y. P., Wood, A. & Maris, J. M. Inhibition of ALK signaling for cancer therapy. Clin. Cancer Res. 15, 5609–5614 (2009).
Wang, Y. W. et al. Identification of oncogenic point mutations and hyperphosphorylation of anaplastic lymphoma kinase in lung cancer. Neoplasia 13, 704–715 (2011).
Murugan, A. K. & Xing, M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 71, 4403–4411 (2011).
Grzelinski, M. et al. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia 11, 145–156 (2009).
van Gaal, J. C. et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J. Clin. Oncol. 30, 308–315 (2012).
Englund, C. et al. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature 425, 512–516 (2003).
Mathivet, T., Mazot, P. & Vigny, M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal. 19, 2434–2443 (2007).
Stoica, G. E. et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 276, 16772–16779 (2001).
Stoica, G. E. et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998 (2002).
Bai, R. Y. et al. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 96, 4319–4327 (2000).
Chiarle, R. et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 11, 623–629 (2005).
Kasprzycka, M., Marzec, M., Liu, X., Zhang, Q. & Wasik, M. A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc. Natl Acad. Sci. USA 103, 9964–9969 (2006).
Zou, H. Y. et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 67, 4408–4417 (2007).
Bresler, S. C. et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci. Transl. Med. 3, 108ra114 (2011).
Hallberg, B. & Palmer, R. H. ALK and NSCLC: Targeted therapy with ALK inhibitors. F1000 Med. Rep. 3, 21 (2011).
Kwak, E. L. et al. naplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Sasaki, T., Rodig, S. J., Chirieac, L. R. & Janne, P. A. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur. J. Cancer 46, 1773–1780 (2010).
Knudson, A. G. Jr & Strong, L. C. Mutation and cancer: neuroblastoma and pheochromocytoma. Am. J. Hum. Genet. 24, 514–532 (1972).
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nat. Med. 10, 789–799 (2004).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Caren, H., Abel, F., Kogner, P. & Martinsson, T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem. J. 416, 153–159 (2008).
Chen, Y. et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455, 971–974 (2008).
George, R. E. et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455, 975–978 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
McDermott, U. et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 68, 3389–3395 (2008).
Tabori, U. & Malkin, D. Risk stratification in cancer predisposition syndromes: lessons learned from novel molecular developments in Li-Fraumeni syndrome. Cancer Res. 68, 2053–2057 (2008).
Lamant, L. et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am. J. Pathol. 156, 1711–1721 (2000).
Dirks, W. G. et al. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int. J. Cancer 100, 49–56 (2002).
George, R. et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS ONE 2, e255 (2007).
Bossi, R. T. et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry 49, 6813–6825 (2010).
Lee, C. C. et al. Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem. J. 430, 425–437 (2010).
Sasaki, T. et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 70, 10038–10043 (2010).
Weiser, D. et al. Stratification of patients with neuroblastoma for targeted ALK inhibitor therapy. J. Clin. Oncol. 29 (Suppl. 15), 9514 (2011).
De Brouwer, S. et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin. Cancer Res. 16, 4353–4362 (2010).
Schonherr, C. et al. Activating ALK mutations found in neuroblastoma are inhibited by crizotinib and NVP-TAE684. Biochem. J. 440, 405–413 (2011).
Schonherr, C. et al. The neuroblastoma ALK(I1250T) mutation is a kinase-dead RTK in vitro and in vivo. Transl. Oncol. 4, 258–265 (2011).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Schulte, J. H. et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin. Cancer Res. 17, 5082–5092 (2011).
Passoni, L. et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 69, 7338–7346 (2009).
Duijkers, F. A. et al. Anaplastic lymphoma kinase (ALK) inhibitor response in neuroblastoma is highly correlated with ALK mutation status, ALK mRNA and protein levels. Cell. Oncol. 34, 409–417 (2011).
Cancer Target Discovery and Development Network et al. Towards patient-based cancer therapeutics. Nat. Biotechnol. 28, 904–906 (2010).
Galkin, A. V. et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc. Natl Acad. Sci. USA 104, 270–275 (2007).
Christensen, J. G. et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 6, 3314–3322 (2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Skolnik, J. M., Barrett, J. S., Jayaraman, B., Patel, D. & Adamson, P. C. Shortening the timeline of pediatric phase I trials: the rolling six design. J. Clin. Oncol. 26, 190–195 (2008).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010).
Engelman, J. A. & Settleman, J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr. Opin. Genet. Dev. 18, 73–79 (2008).
Buonamici, S. et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2, 51ra70 (2010).
Carpenter, E. L. et al. Mechanisms of resistance to small molecule inhibition of anaplastic lymphoma kinase in human neuroblastoma. Presented at the 102nd American Association for Cancer Research Conference.
Moog-Lutz, C. et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Biol. Chem. 280, 26039–26048 (2005).
Baselga, J. et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14, 737–744 (1996).
Robert, F. et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J. Clin. Oncol. 19, 3234–3243 (2001).
Regales, L. et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J. Clin. Invest. 119, 3000–3010 (2009).
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Xia, W. et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24, 6213–6221 (2005).
Weiss, W. A., Aldape, K., Mohapatra, G., Feuerstein, B. G. & Bishop, J. M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16, 2985–2995 (1997).
Faisal, A. et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol. Cancer Ther. 10, 2115–2123 (2011).
Terrile, M. et al. miRNA expression profiling of the murine TH-MYCN neuroblastoma model reveals similarities with human tumors and identifies novel candidate miRNAs. PLoS ONE 6, e28356 (2011).
Chayka, O. et al. Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas. J. Natl Cancer Inst. 101, 663–677 (2009).
Teitz, T. et al. Preclinical models for neuroblastoma: establishing a baseline for treatment. PLoS ONE 6, e19133 (2011).
Chesler, L. et al. Chemotherapy-induced apoptosis in a transgenic model of neuroblastoma proceeds through p53 induction. Neoplasia 10, 1268–74 (2008).
Hogarty, M. D. et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 68, 9735–9745 (2008).
Chesler, L. et al. Malignant progression and blockade of angiogenesis in a murine transgenic model of neuroblastoma. Cancer Res. 67, 9435–9442 (2007).
Cheng, A. J. et al. Cell lines from MYCN transgenic murine tumours reflect the molecular and biological characteristics of human neuroblastoma. Eur. J. Cancer 43, 1467–1475 (2007).
Burkhart, C. A. et al. Effects of MYCN antisense oligonucleotide administration on tumorigenesis in a murine model of neuroblastoma. J. Natl Cancer Inst. 95, 1394–1403 (2003).
Chanthery, Y. H. et al. Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Sci. Transl. Med. 4, 115ra3 (2012).
Chesler, L. & Weiss, W. A. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin. Cancer Biol. 21, 245–255 (2011).
Etchin, J., Kanki, J. P. & Look, A. T. Zebrafish as a model for the study of human cancer. Methods Cell Biol. 105, 309–337 (2011).
Ozanne, B., Richards, C. S., Hendler, F., Burns, D. & Gusterson, B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J. Pathol. 149, 9–14 (1986).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Han, S. W. et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 23, 2493–2501 (2005).
Acknowledgements
This work is supported in part by grants from the National Institute of Health (R01-CA140198), the Children's Oncology Group, the Carly Hillman Fund and the US Army Peer-Reviewed Medical Research Program (W81XWH-10-1-0212/3) granted to Y. P. Mossé.
Author information
Authors and Affiliations
Contributions
Both authors made a substantial contribution to researching data for the article, discussion of content, and to writing, revising and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
Y. P. Mossé declares that she has received research funding from Pfizer. E. L. Carpenter declares no competing interests.
Rights and permissions
About this article
Cite this article
Carpenter, E., Mossé, Y. Targeting ALK in neuroblastoma—preclinical and clinical advancements. Nat Rev Clin Oncol 9, 391–399 (2012). https://doi.org/10.1038/nrclinonc.2012.72
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.72
This article is cited by
-
Comprehensive exploration of the involvement of cuproptosis in tumorigenesis and progression of neuroblastoma
BMC Genomics (2023)
-
Genomic ALK alterations in primary and relapsed neuroblastoma
British Journal of Cancer (2023)
-
Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas
Endocrine Pathology (2022)
-
Clinical cancer genomic profiling
Nature Reviews Genetics (2021)
-
Protein tyrosine phosphatase PTPN1 modulates cell growth and associates with poor outcome in human neuroblastoma
Diagnostic Pathology (2019)