Abstract
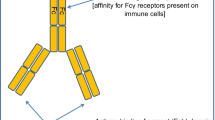
United Therapeutics Corporation and the National Cancer Institute are developing dinutuximab (Unituxin™; ch14.18), a monoclonal antibody targeting GD2, for the treatment of neuroblastoma. GD2 is a glycolipid found on the surface of tumour cells, which is overexpressed in neuroblastoma. Dinutuximab, an IgG1 human/mouse chimeric switch variant of murine monoclonal antibody 14G2a, binds to GD2 and induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. The US FDA has recently approved the use of dinutuximab combination therapy for the treatment of high-risk neuroblastoma in paediatric patients. The marketing authorization application for dinutuximab is under regulatory review in the EU, and phase I–III development is underway in several other countries. This article summarizes the milestones in the development of dinutuximab leading to this first approval for use (in combination with granulocyte macrophage colony-stimulating factor, interleukin-2 and 13-cis retinoic acid) in the treatment of paediatric patients with high-risk neuroblastoma who achieve at least partial response to prior first-line multiagent, multimodality therapy.
Similar content being viewed by others
References
Hara J. Development of treatment strategies for advanced neuroblastoma. Int J Clin Oncol. 2012;17(3):196–203.
Castel V, Segura V, Canete A. Treatment of high-risk neuroblastoma with anti-GD2 antibodies. Clin Transl Oncol. 2010;12(12):788–93.
Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma: the role of monoclonal antibodies. Ann Pharmacother. 2013;47(2):210–8.
Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 2014;588(2):288–97.
Mueller BM, Romerdahl CA, Gillies SD, et al. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144(4):1382–6.
United Therapeutics Corporation. Unituxin™: US prescribing information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125516s000lbl.pdf. Accessed 25 Mar 2015.
US Food and Drug Administration. Dinutuximab. 2015. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm437480.htm. Accessed 16 Mar 2015.
Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34.
Albertini MR, Hank JA, Schiller JH, et al. Phase Ib trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3(8):1277–88.
Murray JL, Kleinerman ES, Jia SF, et al. Phase Ia/Ib trial of anti-GD2 chimeric monoclonal antibody 14.18 (ch14.18) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in metastatic melanoma. J Immunother. 1996;19(3):206–17.
Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antibodies Hybridomas. 1992;3(1):19–24.
Choi BS, Sondel PM, Hank JA, et al. Phase I trial of combined treatment with ch14.18 and R24 monoclonal antibodies and interleukin-2 for patients with melanoma or sarcoma. Cancer Immunol Immunother. 2006;55:761–74.
United Therapeutics Corporation. United therapeutics receives a rare pediatric disease priority review voucher following the approval of Unituxin™ for pediatric high-risk neuroblastoma (media release). 2015. http://ir.unither.com/releasedetail.cfm?ReleaseID=900822.
Department of Health and Human Services. BLA 125516 (BLA approval). 2015. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/125516Orig1s000ltr.pdf. Accessed 1 Apr 2015.
Barker E, Mueller BM, Handgretinger R, et al. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51(1):144–9.
Uttenreuther-Fischer MM, Huang CS, Yu AL. Pharmacokinetics of human-mouse chimeric anti-GD2 mAb ch14.18 in a phase I trial in neuroblastoma patients. Cancer Immunol Immunother. 1995;41(6):331–8.
Desai AV, Fox E, Smith LM, et al. Pharmacokinetics of the chimeric anti-GD2 antibody, ch14.18, in children with high-risk neuroblastoma. Cancer Chemother Pharmacol. 2014;74(5):1047–55.
Ozkaynak MF, Gilman AL, Yu AL, et al. A comprehensive safety trial of chimeric antibody 14.18 (ch14.18) with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: a Children’s Oncology Group study (abstract no. 10044). J Clin Oncol. 2014;32(15 Suppl 1).
Handgretinger R, Anderson K, Lang P, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31a(2):261–7.
Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–80.
Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside GD2 monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18(24):4077–85.
Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27(1):85–91.
Simon T, Hero B, Faldum A, et al. Infants with stage 4 neuroblastoma: the impact of the chimeric anti-GD2-antibody ch14.18 consolidation therapy. Klin Padiatr. 2005;217(3):147–52.
Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22(17):3549–57.
Simon T, Hero B, Faldum A, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. Dhillon is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Dhillon, S. Dinutuximab: First Global Approval. Drugs 75, 923–927 (2015). https://doi.org/10.1007/s40265-015-0399-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0399-5