Article Text
Abstract
Background Intradiaphragmatic extralobar pulmonary sequestration (IDEPS) is a rare type of pulmonary sequestration (PS). The purpose of this study is to assess diagnosis and operative treatment of IDEPS.
Methods Patients with PS who were diagnosed and treated in our center from January 2015 to December 2020 were analyzed retrospectively to identify patients with IDEPS.
Results Totally, 215 patients with PS were treated surgically, including 10 cases with IDEPS. Prenatal ultrasounds and postnatal-enhanced CT showed the presence of IDEPS in four cases and in seven cases, respectively. The three-dimensional (3D) reconstruction software was performed perfectly to identify the location of the lesions in 10 cases. The surgeries were performed smoothly by laparoscopic surgery in one case, video-assisted thoracic surgery (VATS) in five cases and Da Vinci robot-assisted thoracoscopic surgery (DVRATS) in four cases. In the VATS group, the average operative duration, intraoperative blood loss volume, length of stay after operation, and postoperative thoracic catheter indwelling duration were 48 min, 3.8 mL, 6.4 days and 2.2 days, respectively. That of the DVRATS group were 80 min, 3.5 mL, 4.3 days and 1.5 days, respectively. No side effects had appeared.
Conclusions The 3D reconstruction software was proven to be capable in assisting the assessment of IDEPS. We suggested early surgery to treat IDEPS, and the best path was accessing the mass from the chest. Both DVRATS and VATS for the treatment of an IDEPS are safe, feasible, and effective. Furthermore, DVRATS provides a 3D magnified view, more flexibility and precision.
- pediatrics
- thoracic surgery
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Intradiaphragmatic extralobar pulmonary sequestration (IDEPS) is a rare entity in pediatric surgical practice.
It is challenging to diagnose an IDEPS using prenatal ultrasound or postnatal CT; video-assisted thoracic surgery (VATS) has been used for the treatment of an IDEPS.
What are the new findings?
Three-dimensional (3D) reconstruction software was accurate to assist on the diagnose of an IDEPS.
Da Vinci robot-assisted thoracoscopic surgery (DVRATS) is safe, feasible and effective for the treatment of an IDEPS.
Compared with VATS, DVRATS provides a 3D magnified view, more flexibility and precision.
How might it impact on clinical practice in the foreseeable future?
In children with a mass located at the diaphragm, surgeons should consider the possibility of IDEPS, and misdiagnosis should be avoided before surgery as much as possible. DVRATS is a more helpful method for management of an IDEPS.
Introduction
Pulmonary sequestration (PS) is a rare congenital pulmonary development anomaly in infants and children. PS is defined as pulmonary tissue lacking of tracheobronchial connection but with systemic arterial supply, and it occurs in approximately 0.15%~6.4% of all pulmonary malformations.1
According to the presence or absence of visceral pleura, PS is classified as two types: intralobar pulmonary sequestration (IPS) and extralobar pulmonary sequestration (EPS). IPS usually appears at the lower lobe of both lungs, especially at the left lower lobe. EPS, which accounts for 0.1%–1.8% of cases, is the second most common lung malformation in children.2 It mainly appears between the left lower lobe of the lung, especially near the mediastinal spine,3 but occasionally occurs at the inner part of the diaphragm, abdomen and neck.3–6 In some cases, IPS and EPS may coexist.3 4 In EPS, intradiaphragmatic extralobar pulmonary sequestration (IDEPS) mostly occurs on the left,3 5 was first reported by Caulet in 1962, and accounts for 13%–18% of the PS.5 7
The rate of infection within IDEPS has been reported to be 16.7%.3 The infection may cause diaphragmatic edema, adhesion and elevation, making it more difficult to separate the diaphragm fibrous tissue from the mass. In addition, respiratory function may be affected by the elevation of diaphragm; hence, early surgical management of IDEPS is necessary. In the present study, we performed a retrospective examination of ten such cases and summarized experiences in the diagnosis and surgical treatment.
Methods
Patients
Data from patients with PS who underwent surgery in our institution from January 2015 to December 2020 were collected retrospectively. The diagnosis of patients with IDEPS was performed by postnatal-enhanced computed tomography (CT). Surgeries were performed successfully to manage IDEPS, including video-assisted thoracic surgery (VATS), Da Vinci robot-assisted thoracoscopic surgery (DVRATS) and laparoscopic surgery (LS). The surgical indication for VATS or DVRATS is that patients must be in physical condition that can withstand the cardiorespiratory changes associated with this treatment. The presence of pleural adhesions was not considered as an absolute contraindication anymore. Besides, we preferred the age of children over 1 year who underwent DVRATS due to the space requirement of the robot arm. However, the age of operation may be flexible according to the surgeon’s experience. The gender, age, operative duration, intraoperative blood loss volume, length of stay after operation, postoperative thoracic catheter indwelling duration and postoperative side effect were documented in this condition.
Surgery techniques
Da Vinci robot-assisted thoracoscopic surgery
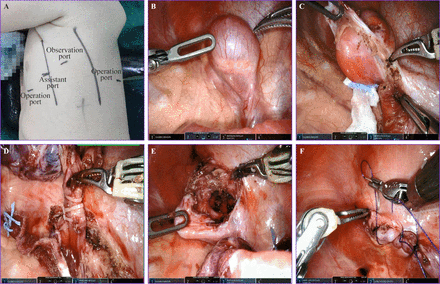
Operations were performed using routine tracheal intubation, general anesthesia and single-lung ventilation. Each patient was positioned in the lateral decubitus position with the mass side facing up, and a pad was positioned to a slight widening of the intercostal space. Four incisions were performed (figure 1A): an observation port (8 mm) was placed in the fourth intercostal space at the mid-axillary line, two operation ports (8 mm) were placed in the sixth intercostal space at the mid-clavicular line, and the seventh intercostal space at the subscapular line, respectively. The remaining 5 mm port (assistant port) was placed in the fifth intercostal space at the anterior axillary line. During the operation, a mass protruding from the diaphragm to the chest cavity could be found (figure 1B). The diaphragm was opened along the edge of the mass, and the mass was completely resected (figure 1C and E). The nutrient arteries were ligated with 3-0 silk thread or clipped with vascular clamp (figure 1D). Then, the diaphragm was repaired with interrupted 3-0 barbed suture (figure 1F). Lastly, a thoracic drainage tube was routinely placed, and the operation was completed. If the single-lung ventilation failed, the pressure of CO2 was maintained at 6~8 mmHg to produce an artificial pneumothorax.
Intradiaphragmatic extralobar pulmonary sequestration in a nine-month-old girl treated with Da Vinci robot-assisted thoracoscopic surgery. (A) Locations of the incision; (B) A mass protruding from the diaphragm to the chest cavity; (C) The diaphragm was opened along the edge of the mass; (D) The nutrient arteries were ligated with vascular clamps; (E) Mass was completely resected; (F) Diaphragm was repaired with interrupted 3-0 barbed suture. (Patient informed consent had been obtained from her parents).
Video-assisted thoracoscopic surgery
Routine tracheal intubation, general anesthesia and single-lung ventilation. The patient was positioned in the lateral decubitus position with the mass side facing up and a pad was positioned to a slight widening of the intercostal space. Three incisions were performed: an observation port (5 mm) was placed in the eighth intercostal space at the posterior axillary line. Two operation ports (5 mm) were placed in the sixth intercostal space at the anterior axillary line, and eighth intercostal of subscapular line. The operation method is the same as above.
Laparoscopic surgery
Routine tracheal intubation combined with general anesthesia. The patient was in the supine position with a pad to slightly elevate the abdomen. Three incisions were performed: an observation port (5 mm) was placed in the navel. Two operation ports (5 mm) were placed in the 4 cm above umbilicus and the right abdomen. The operation method is the same as above. The pressure of CO2 was maintained at 6~8 mmHg to produce an artificial pneumothorax.
Results
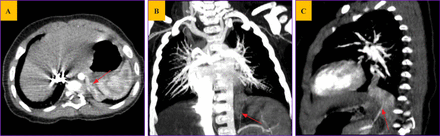
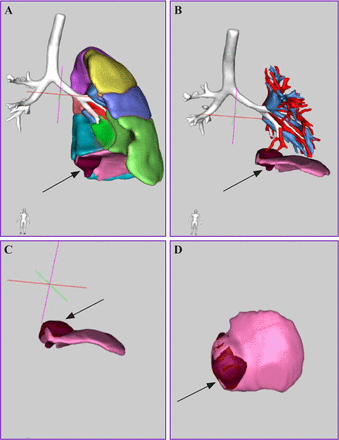
A total of 215 patients with PS were enrolled from January 2015 to December 2020 in our hospital, including 128 patients with IPS and 87 patients with EPS. Ten patients (11.5%) with IDEPS were identified (2 boys, 8 girls; age, 5–21 months, mean age, 10.1±4.6 months) (table 1), and 1 case complicated with pectus excavatum. All IDEPSs were entirely inside the left diaphragm and asymptomatic all the time. All cases except one were discovered first in prenatal ultrasound (US); 4 cases were diagnosed as IDEPS; 5 cases were diagnosed as IDEPS upper-diaphragmatic EPS, intraperitoneal tumor, or retroperitoneal tumor; and 1 case was discovered occasionally at operation. The prenatal US detected an aberrant vessel in seven cases. Postnatal-enhanced CT was performed routinely after birth and showed the presence of IDEPS in seven cases (figure 2). One patient was diagnosed with an infection in the IDEPS and was treated successfully by surgery 1 month after anti-infection treatment for 2 weeks. Meanwhile, a three-dimensional (3D) reconstruction software (The Hexalotus company, Su Zhou, China) was performed to identify the location of the lesions in 10 cases (figure 3). Full diaphragm and the mass can be automatically modelled by 3D reconstruction software. The aberrant nutrient arteries included a blood supply via the thoracic aorta in one case, via the splenic artery in one case, via the abdominal aorta in four cases, and via the small artery branches in four cases.
Enhanced computed tomography (CT) demonstrated the presence of a solid mass at the left diaphragm area. A single systemic artery (red arrows) arising from the abdominal aorta was seen to supply the mass. (A) Horizontal CT image; (B) Coronal CT image; (C) Sagittal CT image.
Three-dimensional (3D) reconstruction imaging of a patient with intradiaphragmatic extralobar pulmonary sequestration. The black arrows showed in figure (A, B, C, D)marked a mass (dark red area) located in the diaphragm. Here we introduced medical image-processing software, which could reconstruct the patient’s CT images into 3D data set using a specialized algorithm, and we have obtained permission from the company.
Characteristics of IDEPS cases
VATS, DVRATS and LS had been performed in 5 cases, 4 cases and 1 case, respectively. One case (male; age, 21 months) was treated with LS lasted about 120 min, and intraoperative blood loss volume was 10 mL. VATS and DVRATS were defined as VATS group and DVRATS group. In the VATS group (five girls; mean age, 8.4 months), the average operative duration, intraoperative blood loss volume, length of stay after operation, and postoperative thoracic catheter indwelling duration were 48 min, 3.8 mL, 6.4 days and 2.2 days, respectively. In the DVRATS group (1 boy, 3 girls; mean age, 9.5 months), the average operative duration, intraoperative blood loss volume, length of stay after operation, and postoperative thoracic catheter indwelling duration were 80 min, 3.5 mL, 4.3 days and 1.5 days, respectively. The diagnosis of IDEPS was confirmed in 10 cases according to the histopathological results (figure 4), and inflammatory cell infiltration on pathologic specimen was found in 3 cases. Patients with IDEPS who were followed up from 3 months to 36 months were included in this retrospective study, and all the results from these check-ups were normal.
Pathological specimen of a patient with intradiaphragmatic extralobar pulmonary sequestration. The section of lesion exhibited a honeycomb-like appearance.
Discussion
PS was first reported by Rokitansky in 1861, and the etiology and pathogenesis of this disease remain unclear,8 which may be related to the imbalance of cell proliferation and apoptosis.9 Compared with congenital cystic adenomatoid malformation, aberrant feeding arteries of PS mainly arise from the thoracic aorta, followed by the abdominal aorta, intercostal artery, and subclavian artery.3 10 Studies3 11 have shown that PS was associated with many deformities, such as diaphragmatic hernia, congenital heart disease, cyst of bronchial origin, and funnel chest (pectus excavactum or pectus carinatum). With the popularization of prenatal examination, PS has increasingly been detected by prenatal US or has been found incidentally on postnatal CT imaging. However, it is still challenging to identify an IDEPS. The study12 found that the rise of PS during the 9th–12th weeks of embryonic development may have a higher chance to form into the diaphragm. The clear diagnosis of an IDEPS was conducted mainly by prenatal US, enhanced CT, or magnetic resonance imaging (MRI), which play important roles in the surgical planning and safe operative resection. Prenatal US is a preferred method for the diagnosis of fetal PS.13 However, due to the special location of a sequestered mass in diaphragm, it is difficult to clearly diagnose whether mass is located in the diaphragm using prenatal US. The detection rate of fetal IDEPS, which proved this difficulty, was only 40% in our study. MRI is a valuable method in diagnosing IDEPS. MRI can provide multilevel anatomical location information of lesions and is superior to prenatal US in assessing the feeding arteries and venous.14 However, the diaphragm may be affected by respiratory activity, limiting the application of MRI to identify an IDEPS. Given the limitations of MRI, a CT scan is less affected by respiratory activity and has a higher concordance rate for characterizing the lung parenchyma. In particular, the reformatted imaging CT scan can demonstrate the presence of that mass was enveloped by the diaphragm. In addition, CT is the most accurate method for the detection of a systemic vasculature to the mass. Although CT scan has the risk of ionizing radiation, it is the most efficient choice among the other methods for identifying an IDEPS. Chun et al5 reported that 11 cases of IDEPSs had been confirmed after birth using enhanced CT, but Olivieri et al15 suggested that a diagnosis could only be confirmed at surgery. In our institution, the concordance rate of CT was 70%, and the feeding thoracic aorta and abdominal aorta account for 50%, which were lower compared with previous studies.5 16 17 The 3D reconstruction software was used to provide access to more and higher-quality information about a patient’s 3D anatomy, making it possible to improve the accuracy and reliability of diagnosis and treatment. This seems to be the first report that a 3D reconstructed imaging clearly showed the relationship between mass and diaphragm and helped to identify an IDEPS.
The ideal proper management of PS remains controversial. Some studies18–20 demonstrated that an EPS may remain asymptomatic throughout life and even involute over time, suggesting the safety of just following up EPS without surgical management. In contrast, a majority of surgeons suggested PS should be surgically resected,1 3 11 18 and surgical resection should be performed no more than 12 months of age.3 18 Trabalza Marinucci et al1 reported that pediatric patients operated after 1 year of age developed more respiratory symptoms than those treated before 1 year of age. Stanton8 found that the rate of postoperative complications of symptomatic PS was significantly higher than that of asymptomatic patients with PS undergoing elective surgery. Currently, VATS has become the main surgical technique with the advantages of being minimally invasive, accurate and rapid recovery. Some studies21–23 reported the application of transcatheter arterial embolization in the treatment of PS. This method is not widely practiced and has many complications (especially ionizing radiation). The method also needs a larger sample size and multiple centers of data to be evaluated more precisely. The IDEPS is rarely reported.3 5 7 12 16 24 25 It has the risk of infection,3 lung ventilation disorder24 or malignancy.3 5 In addition, once an infection occurs, the surgical difficulty is significantly increased owing to the adhesions and edema of the diaphragm, which may result in more complications.5 25 Owing to the special location of an IDEPS, it is difficult to differentiate from upper-diaphragmatic EPS, neuroblastoma, adrenal tumor or teratoma. Relying solely on morphological diagnosis does not fully determine the nature of the abnormal mass. Meanwhile, the non-operative management of IDEPS should monitor the sizes and infection by CT or by changes in clinical symptoms. Nevertheless, the convenience and effectiveness of these follow-up processes are not ensured or precise. Considering all the conditions, we conducted surgery of all IDEPS from the chest. During surgery, the aberrant feeding vessels and the ruptured diaphragm should be properly managed to avoid massive bleeding and diaphragm expansion. One case who was treated with VATS in our center suffered from infection, making it difficult to identify the feeding vessels, dissociate the tissue and resect the lesion, all of which led to increased operative duration and postoperative thoracic catheter indwelling duration. One case was performed successfully by LS. However, it was difficult to find the mass during the operation, resulting in the prolongation of the operative duration.
To our knowledge, this is the first report of DVRATS for the treatment of an IDEPS. Compared with VATS, DVRATS could allow a 3D magnified view of the surgical field (10–15 times magnification power), eliminate hand tremors, and provide a wider range of motion to the surgical performance, all of which make it easier to complete ligation of the feeding vessel, resection of the mass and subtle suture. In addition, we found that the length of stay after operation and postoperative thoracic catheter indwelling duration in the DVRATS group were significantly shorter than those of the VATS group. However, owing to the limited experience and long preparation time, the first case managed with DVRATS consumed more time than the other cases. The limitations of expensive equipment, high cost and complete absence of touch sensation restrict its wide application.
In conclusion, IDEPS is a rare congenital anomaly, which is most commonly located at the left diaphragm. Misdiagnosis should be avoided before surgery as much as possible. Although it is challenging to diagnose an IDEPS using prenatal US or CT, enhanced CT is an essential technique for identifying an IDEPS. This study applied a relatively new technique of 3D reconstruction software to appropriately assist on the assessment of an IDEPS. Early surgery to resect the IDEPS was recommended, and the best path was accessing the mass from the chest. The treatment of an IDEPS with DVRATS is as safe, feasible and effective as VATS. Furthermore, compared with VATS, DVRATS provides a 3D magnified view and more flexibility and precision. Small sample size may affect results, so further clinical studies with more cases are needed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Ethics Board of the Children's Hospital, Zhejiang University School of Medicine on June 21, 2021 (approval number: 2021-1RB-151).
References
Footnotes
Contributors YG was responsible for conceptualization, data curation, formal analysis, software, supervision, project administration, writing (original draft), and writing (review). XH contributed to data curation, formal analysis, resources, software, methodology, validation, visualization, and writing (original draft). JJ performed supervision and writing (review and editing). ZT performed formal analysis, methodology, supervision, and writing (review and editing). YG is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.