Article Text
Abstract
Background Thoracoabdominal neuroblastoma is a unique surgical challenge. We reviewed our experience focusing on disease patterns and corresponding surgical approaches.
Methods Among 310 patients in our neuroblastoma database, 30 (9.7%) had thoracoabdominal neuroblastoma. Patients’ clinical charts were reviewed and analyzed. Two disease patterns were identified: solitary thoracoabdominal tumor (group A, n=15) and multifocal tumors in thorax and abdomen (group B, n=15). Operative approaches were categorized based on routes of surgical access.
Results Thirty patients with average age of 4.1 (range 0.8–12.8) years were studied. All received preoperative chemotherapy. Among 15 group A patients, four were stage 3 intermediate risk (IR) and 11 were stage 4 high risk (HR). Surgical approaches included single-incision thoracoabdominal approach (n=10), laparotomy-cum-transdiaphragmatic approach (n=3), and laparotomy-cum-thoracotomy approach (n=2). One patient had 10% residual disease and the rest achieved gross total resection. Postoperative complications included chylous ascites (n=3), intestinal obstruction (n=3), pneumonia (n=1), spinal cord infarction (n=1) scoliosis (n=2) and thoracoabdominal nerves palsy (n=3). Among 15 group B patients, all were stage 4 with five IR and 10 HR. Thoracic components were found in the posterior mediastinum (n=7), superior mediastinum (n=4), middle mediastinum (n=1), parietal pleura (n=3) and lungs (n=3). Surgical approaches included multi-incision laparotomy-cum-thoracotomy (n=14) and laparotomy-cum-transdiaphragmatic approach (n=1). Gross total resection was achieved in all surgeries. Postoperative complications included chylous ascites (n=3). Overall, all nine IR patients survived without evidence of disease and 9 (42.8%) HR patients died of disease. There was no perioperative mortality.
Conclusion Surgical resection of thoracoabdominal neuroblastoma is feasible and safe. Despite its complexity, thoracoabdominal neuroblastoma has comparable treatment outcomes when compared with single-compartmental disease.
- neuroblastoma
- thoracoabdominal
- surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Thoracoabdominal neuroblastoma involves dual compartments.
It is an image-defined risk factor.
It is uncommon.
Surgery is complex and challenging.
What are the new findings?
Surgical management should be performed after neoadjuvant chemotherapy.
Multiple surgical approaches are available.
Thoracoabdominal neuroblastoma had comparable treatment outcomes when compared with single-compartmental disease.
How might it impact on clinical practice in the foreseeable future?
The availability of various surgical approaches provides options to successfully treat this group of tumors.
Introduction
Neuroblastoma involving the thorax and abdomen concurrently is a unique surgical challenge. Involving two major body compartments, these tumors are usually extensive manifesting as solitary or multifocal tumors.
Ipsilateral solitary tumor extension within two body compartments, such as thorax and abdomen, has been defined by the International Neuroblastoma Risk Group (INRG) as an image-defined risk factor (IDRF).1 IDRFs are features detected on imaging that make safe and total tumor excision impracticable at the time of diagnosis. Such solitary thoracoabdominal (TA) tumors should be distinguished from multifocal tumors that may represent either disseminated metastatic disease outside the primary tumor’s body compartment or multifocal primary tumors.2 Each tumor group has unique anatomical characteristics with surgical and prognostic implications.
Surgery for TA neuroblastoma may require surgeons with different expertise, multiple incisions and multistaged operations. Surgical approaches may vary among patients and surgeons. We reviewed our experience in TA neuroblastoma focusing on the disease patterns and the corresponding surgical approaches.
Methods
A retrospective review was conducted in our neuroblastoma database. Informed consent was not required for this study. Among 310 patients who underwent surgical treatment from year 2005 to 2018, thirty (9.7%) had neuroblastoma that involved both thorax and abdomen concurrently. Patients’ demography, disease status, preoperative imaging studies, operative reports and follow-up clinical charts were analyzed. Patients were staged according to International Neuroblastoma Staging System (INSS),3 the Children’s Oncology Group risk stratification system and the INRG Staging System (INRGSS).1
Two disease patterns were identified: solitary TA tumor (group A, n=15) and multifocal tumors in thorax and abdomen (group B, n=15). The former comprised solitary primary tumors that cross-occupied both thoracic and abdominal compartments while the latter consisted of multiple independent tumors distributed in the two compartments. All patients received preoperative chemotherapy with a surgical goal of complete removal of primary tumor without mutilation. High-quality axial imaging by CT provided important anatomic detail to plan the extent of tumor resection. It was our routine practice to remove all resectable non-primary disease such as those found in regional or distant lymph nodes, paravertebral region, pleural region, lungs and liver for the purpose of reducing tumor burden. The technique of subadventitial tumor dissection was applied for tumors with vascular encasement.4 The operative approaches were selected based on tumors’ locations and surgeon’s preference and were categorized according to the routes of surgical access. The duration of surgery, associated complications and outcome were analyzed. Statistical analysis was performed using Fisher’s exact test and Student’s t-test. Survival curves were calculated according to Kaplan-Meier. Survival times were compared by the log-rank test. Overall survival (OS) was calculated as the time from diagnosis to death or last examination.
Our surgical team operated at a tertiary referral center for pediatric surgical oncology for both local and overseas hospitals, and that may have influenced the types of patients received. We managed patients from various institutions with diverse treatment regimens, hence limiting the interpretation of outcome statistics. Ten (33.3%) patients received all or partial preoperative chemotherapy at our institution. After surgery, 12 (40%) patients received consolidation therapy at our institution while the rest returned to their referring institutions.
Results
Thirty patients with average age of 4.1 (range 0.8–12.8) years, with 15 males and 15 females, were studied. They received between 2 and 11 (median 5.5) cycles of preoperative chemotherapy. Twenty-six (86.7%) patients had metastatic disease of which 21 had high-risk (HR) disease and five had intermediate-risk (IR) disease. Four patients had stage 3 IR disease (table 1). Mean follow-up was 4.9 (range 0.4–14.2) years.
Comparison of characteristics of groups A and B
Among 15 group A patients, 11 (73.3%) were stage 4 HR while 4 (26.7%) were stage 3 IR. All patients had primary tumors that arose from non-adrenal sympathetic chain. Ten had predominantly left-sided tumors while five had right-sided tumors. All had at least three IDRFs, namely ipsilateral tumor extension within two body compartments, lower mediastinal tumor infiltrating the costovertebral junction between T9 and T12 and tumor infiltrating the diaphragm. Other IDRFs included tumor encased aorta (n=10), intraspinal tumor extension with more than one-third of spinal canal in axial plane invaded (n=4), tumor encasement of celiac axis (n=7), superior mesenteric artery (n=5) and renal pedicles (n=6) and infiltration of the porta hepatis (n=1). They received between 2 and 11 (median 4) cycles of chemotherapy before surgery. However, only 4 (26.7%) patients showed tumor size reduction, with Response Evaluation Criteria in Solid Tumors >30% of the longest dimension after chemotherapy.5 The rest remained as massive tumors that posed challenges to surgical resection. Among the 11 stage 4 HR patients, four also underwent resection of supraclavicular lymph nodes and one underwent resection of ipsilateral axillary lymph nodes.
All underwent single-staged surgery. Surgical approaches included TA approach (n=10), laparotomy-cum-transdiaphragmatic approach (n=3), and laparotomy-cum-thoracotomy (n=2).
Transdiaphragmatic approach was feasible when the thoracic component was only in the lower thorax (up to T9 level) and was non-bulky. Our three cases were performed through the left hemidiaphragm after completing abdominal tumor resection. The diaphragm was incised at its posteromedial border followed by detachment of the crus from the underlying aorta. Adequate exposure of the abdominal aorta then guided the tumor dissection along the descending thoracic aorta.
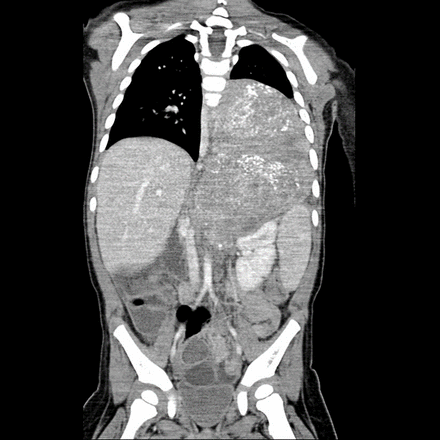
In the presence of bulky tumors that obstructed and limited access, larger TA or multi-incisional exposures were applied (figure 1). Subcostal laparotomy with independent posterolateral thoracotomy was performed in two patients. In both patients, the abdominal incision had to be closed and the patient repositioned before thoracotomy was performed, hence lacked continuity from the abdominal surgery. In recent years, the TA approach was preferred as it provided better exposure.
CT scan of a large thoracoabdominal tumor on the left side.
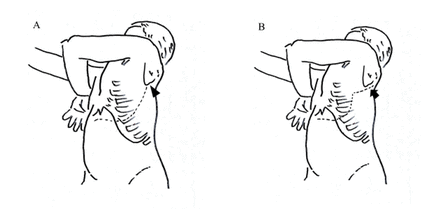
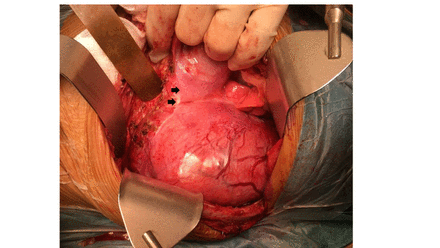
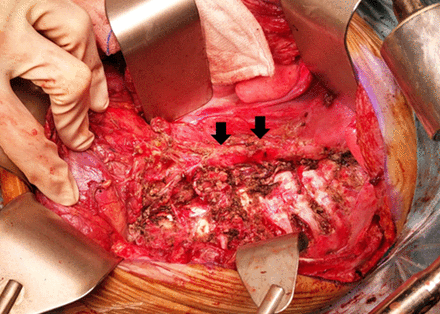
Among the 10 patients who underwent single-incision TA approaches, six were left sided and four were right sided. Six patients underwent TA approach as described by Qureshi and Patil.6 After adopting a semilateral position, a transverse upper abdominal incision was made that traversed obliquely to extend into the eighth intercostal space before reaching the inferior angle of the scapula (figure 2A). Such an incision often limited access to the upper thorax above the aortic arch. An ‘S’ modification was necessary in four patients when tumors extended into the upper thorax. After they were placed in a lateral decubitus position, a posterolateral thoracotomy incision was first made at the fourth intercostal space that extended posteriorly along the medial border of the scapula enabling adequate access to the upper thorax (figure 2B). Anteriorly, the incision continued inferiorly as a rib-cutting vertical incision along the mid-axillary line followed by a transverse upper abdominal incision. Circumferential division of the diaphragm proceeded to connect the thoracic and abdominal cavities in all patients. The tumors were then resected accordingly (figures 3 and 4). At the conclusion of the procedure, the diaphragms were repaired primarily with mattress non-absorbable polypropylene sutures in all patients except one who required polypropylene mesh reconstruction of a large right diaphragmatic defect. Transected ribs were approximated with an overlap and were held together with absorbable polyglactin suture sewn into the adjacent intercostal muscles.
(A) Oblique thoracoabdominal incision (broken line) performed along the eighth intercostal space limited superiorly by the inferior angle of scapula (arrowhead). (B) Thoracoabdominal incision with ‘S’ modification. The upper segment of incision extended along the medial border of scapula (arrow).
Left thoracoabdominal approach to large thoracoabdominal tumor. Arrows denote edge of diaphragmatic division.
Left thoracic cavity after tumor was removed. Descending aorta denoted by arrows.
Average duration of operations was 10.9 (range 5–20) hours. All patients received gross total resection except one with 10% residual disease at the left lower thoracic periaortic region. Eleven patients were extubated immediately after the procedure while four were ventilated overnight. Average intensive care unit (ICU) stay was 0.9 (range 0–2) day. No patients required inotropic support. Postoperative analgesia was provided with morphine infusion for less than 72 hours.
Postoperative complications included chylous ascites (n=3), intestinal obstruction (n=3), pneumonia (n=1), spinal cord infarction (n=1), scoliosis (n=2) and TA nerves palsy (n=3). Among the three patients with chylous ascites, two required tube drainage and supportive dietary management while one underwent surgical repair. Among the three patients with intestinal obstruction one underwent laparoscopic adhesiolysis on postoperative day 39. The patient with pneumonia had a paralyzed right hemidiaphragm that was reconstructed with mesh. The patient with spinal cord infarction developed paralysis of lower limbs soon after her TA surgery. MRI confirmed spinal cord infarction below T8 level consistent with the damage of the Adamkiewicz artery. Despite completing her treatment with peripheral stem cell transplantation, she relapsed with multiple bone metastases 10 months after her surgery. All four IR patients completed chemotherapy according to IR protocol and were in remission. Of the 11 HR patients, 8 underwent peripheral stem cell transplantation, 5 received ch14.18 immunotherapy and 8 underwent radiotherapy. Six (54.5%) relapsed and died of disease and one patient died of sepsis while in remission, and four patients remained well in remission. The latter four had received peripheral stem cell transplantation, immunotherapy and radiotherapy.
Among the 15 group B patients, all had metastatic disease. Five patients who were diagnosed less than 18 months old were classified as IR group while the rest were HR group. All patients had significant abdominal disease except one who had a large right thoracic paravertebral tumor with a concurrent smaller satellite tumor in the pancreatic tail. The abdominal components were adrenal tumors (unilateral (n=8), bilateral (n=2)) or paravertebral tumors (n=5). Thoracic components were found in the posterior mediastinum (paravertebral (n=4) and subcarinal (n=3)), superior mediastinum (n=4), middle mediastinum (n=1), parietal pleura (n=3) and lungs (n=3). Four patients had multiple thoracic lesions. Among the 15 patients, 13 had other concurrent metastatic sites (bone (n=6), bone marrow (n=4), lungs (n=3), brain (n=1), liver (n=1), skin (n=1), and distant lymph nodes (n=9)). The remaining two had pleural metastasis (age 6 years old) and synchronous paravertebral tumor at T4–T9 (age 15 months old).
Four patients’ paravertebral thoracic tumors were classified as synchronous multifocal primary tumors. Among them, three patients had other concurrent metastatic disease and were treated as such, while a 15-month-old girl with bilateral adrenal tumors and thoracic paravertebral tumor was treated as stage 4 IR.
All 15 had laparotomy: 1 accessed the thorax via transdiaphragmatic approach, 2 via trapdoor anterior thoracotomies and 12 via posterolateral thoracotomies. Average duration of operations was 11.2 (range 5–17) hours. Gross total resection was achieved in all surgeries. All patients were extubated immediately after the procedure. One patient required dopamine infusion for 48 hours. Average ICU stay was 1 (range 0–2) day. Postoperative morphine infusion was less than 72 hours. Three patents developed postoperative chylous ascites. Eight HR patients underwent peripheral stem cell transplantation, one received ch14.18 immunotherapy and nine received radiotherapy. All five stage 4 IR patients were alive without evidence of disease. Of the stage 4 HR patients, three died of disease, one was alive with disease, and one died of an unknown cause while in remission 13 months after surgery.
Overall, all nine IR patients survived without evidence of disease and 9 (42.8%) HR patients relapsed and died of disease. Three-year OS for IR and HR disease was 100% and 50.9%±13.3%, respectively (p=0.035). There was no statistical significance when comparison was made between groups A and B (3-year OS 56.4%±15.2% vs 75.8%±12.7%, p=0.33), and when further comparison was made of HR-only patients of both groups (3-year OS 48%±16.4% vs 53.3%±23.3%, p=0.66). There was no perioperative mortality.
Discussion
TA neuroblastoma is a heterogeneous group of tumors with diverse anatomical features associated with specific technical and prognostic implications.
Solitary TA tumors, the most challenging variety, would typically originate from the sympathetic nervous chains located on the anterolateral aspect of the vertebral bodies. For these tumors to cross-occupy both thoracic and abdominal cavities, the tumors occupied both paradiaphragmatic regions with diaphragmatic infiltration. They would typically involve the lower mediastinum infiltrating the costovertebral junction between T9 and T12 associated with the increased risk of anterior spinal artery syndrome following the damage or obstruction of the Adamkiewicz artery,7 as occurred in one of our patients. The above three features of dual compartment infiltration, diaphragmatic infiltration, and T9–T12 costovertebral involvement were IDRFs consistently found in this group of tumors. Other less frequently associated IDRFs included encasement of the aorta and its branches, and intraspinal extension.
In contrast, multifocal tumors located in thoracic and abdominal cavities simultaneously were less likely to infiltrate the diaphragm and lacked a common pattern of IDRFs. More often these included metastases that involved multiple organs or sites distinct from the primary tumor, such as in the lungs, liver, mediastinal lymph nodes and parietal pleura. However, a few of these may be multifocal primary tumors not easily distinguished from metastatic disease as the tissue of origin remained uncertain even on histology. Only bilateral adrenal primary tumors, multiple paravertebral tumors, and adrenal tumor with paravertebral tumors could be classified as multifocal primary tumors with certainty. Multifocality ought to be considered for its genetic implications.2 8
A single TA incision with diaphragmatic detachment was used in 10 of our patients with solitary TA tumors. This approach provided good exposure of the surgical field that included the tumor, its proximal and distal borders and normal vascular structures beyond the tumor (figure 4). In patients with less bulky left-sided lower mediastinal tumors up to T9 level, a laparotomy with transdiaphragmatic incision would suffice. Such an approach is not recommended on the right side due to the limitations imposed by the liver.
TA resection of neuroblastoma is an extensive operation that involves two major body compartments and may be perceived as systemically debilitating with significant postoperative pain and prone to morbidities. Ross et al observed that 21 of their 88 patients with TA resection of neuroblastoma had required inotropes.9 When compared with other tumor groups, our patients’ requirements of postoperative opioid analgesia, ICU stay and mechanical ventilation were not significantly different, a finding consistent with Qureshi’s report.6 Only one of our patients had required inotropic support. Interestingly, we noticed that most of our postoperative complications were related to the tumors’ location rather than the surgical approach.
Conventional multi-incisional approaches to multifocal tumors in the thorax and abdomen were the most frequently used. Though we have frequently performed our laparotomies and thoracotomies together, they may be planned as staged operations to be performed by different surgical teams if necessary.2 In our experience, thoracic incisions varied according to the tumors’ locations while most abdominal accesses were made by transverse upper abdominal incisions.
The INSS has not considered solitary TA neuroblastoma differently. Therefore, we stage assigned and risk stratified our patients as if the tumor was located in a single compartment. In the INRGSS, in the absence of metastases, this group of tumors would be assigned stage L2 based on the presence of IDRFs and therefore neoadjuvant chemotherapy recommended. Surgical resection of the primary tumor, whether in the presence or absence of metastases, remained our therapeutic goal.
Metastasectomy remains a controversy in neuroblastoma treatment. While many current protocols do not mandate the need for metastasectomy, we believe that excision of residual metastases not eradicated by neoadjuvant chemotherapy would reduce tumor burden and the need for additional therapy, such as radiotherapy in these areas.10 In enabling patients to achieve remission, we rendered them eligible for subsequent consolidation therapy.
Our analysis of treatment outcome was limited by the small number of patients and their heterogeneous adjuvant therapies received. Nevertheless, our IR patients had good outcomes while HR patients had inferior outcomes, consistent with current literature.11–13 In spite of the associated surgical challenges, in the era of multimodal risk-based treatment, patients with TA neuroblastoma have comparable outcomes when compared with single-compartmental disease.
References
Footnotes
Contributors CHC planned the study and conducted the research. The manuscript was written by CHC with significant help from AL.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement There are no data in this work. All data relevant to the study are included in the article or uploaded as supplementary information.